无机材料学报 ›› 2016, Vol. 31 ›› Issue (10): 1073-1080.DOI: 10.15541/jim20160173 CSTR: 32189.14.10.15541/jim20160173
杨广武1,2, 杨瑞霞1,3, 张守超2, 朱 飞2
收稿日期:2016-03-23
修回日期:2016-05-18
出版日期:2016-10-20
网络出版日期:2016-09-23
作者简介:杨广武(1970–), 男, 博士研究生. E-mail: yanggw204@163.com
基金资助:YANG Guang-Wu1,2, YANG Rui-Xia1,3, ZHANG Shou-Chao2, ZHU Fei2
Received:2016-03-23
Revised:2016-05-18
Published:2016-10-20
Online:2016-09-23
About author:YANG Guang-Wu. E-mail: yanggw204@163.com
Supported by:摘要:
利用提拉法生长了掺杂浓度为1.0at%~10.0at%的YVO4:Ce3+单晶, XRD分析显示Ce3+ 的掺入没有改变晶体结构。晶体的激发和发射谱测试表明, 在325 nm激发下YVO4:Ce3+发射出峰值在445 nm的蓝光和620 nm附近的红光。蓝光发光强度随Ce3+浓度增加而增强, 当浓度为8.0at%时达到最强, 10.0at%时出现浓度淬灭, 发光减弱; 红光则随着Ce3+浓度的增加而持续增强。通过实验分析推测蓝光来源于Ce3+电子从激发态2D3/2到基态2F5/2的跃迁, 而红光则是由于V4+的电子能级跃迁而形成的。XPS测试显示部分Ce3+失去电子被氧化成为Ce4+, 失去的电子大部分被V5+捕获形成V4+。V4+的d轨道分裂为三个轨道单态2A1、2B1、2B2和一个轨道简并态2E等4个能级, 基态为2B2。V4+中电子通过能量传递、辐射跃迁和无辐射跃迁等过程, 可以实现波长在620 nm附近的红光发射以及在620 nm激发下的451 nm蓝光上转换发光。实验证实了上转换发光为双光子过程。研究结果对紫外激发下YVO4:Ce3+红、蓝光发光行为提供了理论支撑。
中图分类号:
杨广武, 杨瑞霞, 张守超, 朱 飞. Ce3+浓度对YVO4: Ce3+晶体发光性能的影响[J]. 无机材料学报, 2016, 31(10): 1073-1080.
YANG Guang-Wu, YANG Rui-Xia, ZHANG Shou-Chao, ZHU Fei. Ce Doping Concentration on Luminescence Property of YVO4:Ce3+ Crystals[J]. Journal of Inorganic Materials, 2016, 31(10): 1073-1080.
| Samples | Cell parameter/nm | Cell Volume/nm3 V=a2c | |
|---|---|---|---|
| a=b | c | ||
| YVO4 | 0.712140 | 0.629113 | 0.319050 |
| 1.0at% | 0.712641 | 0.630389 | 0.320148 |
| 2.0at% | 0.712730 | 0.630577 | 0.320323 |
| 3.0at% | 0.712782 | 0.630741 | 0.320453 |
| 5.0at% | 0.712823 | 0.63081 | 0.320525 |
| 8.0at% | 0.713023 | 0.630851 | 0.320725 |
| 10.0at% | 0.713103 | 0.631013 | 0.320880 |
表1 YVO4:Ce3+晶胞参数
Table 1 Cell parameters of YVO4:Ce3+ compound
| Samples | Cell parameter/nm | Cell Volume/nm3 V=a2c | |
|---|---|---|---|
| a=b | c | ||
| YVO4 | 0.712140 | 0.629113 | 0.319050 |
| 1.0at% | 0.712641 | 0.630389 | 0.320148 |
| 2.0at% | 0.712730 | 0.630577 | 0.320323 |
| 3.0at% | 0.712782 | 0.630741 | 0.320453 |
| 5.0at% | 0.712823 | 0.63081 | 0.320525 |
| 8.0at% | 0.713023 | 0.630851 | 0.320725 |
| 10.0at% | 0.713103 | 0.631013 | 0.320880 |
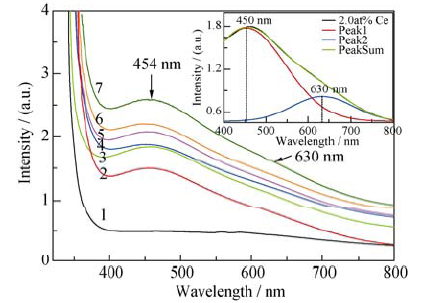
图3 YVO4和YVO4: Ce3+晶体吸收光谱及吸收峰Gauss拟合
Fig. 3 Absorption spectra and Gauss fitting of YVO4 and YVO4: Ce3+ crystals (line 1 to 7 represents absorption spectrum of YVO4 and 1.0at%, 2.0at%, 3.0at%, 5.0at%, 8.0at% and 10.0at% YVO4:Ce3+ crystals, respectively)
| Ce Concentration | Ce3+ Binding energy/eV | Ce4+ Binding energy/eV | Ce3+ Relative content/% | Ce4+ Relative content/% | Ce3+ Rela content/% | Ce4+ Rela content/% |
|---|---|---|---|---|---|---|
| 1.0at% | 891.1,906.8 | 916.3 | 64.8 | 35.2 | 0.613% | 0.333% |
| 2.0at% | 884.2,904.3 | 898.1,916.2 | 75.0 | 25.0 | 1.426% | 0.476% |
| 3.0at% | 886.3,904.8 | 898.1,898.3,915.6 | 65.4 | 34.6 | 1.896% | 1.003% |
| 5.0at% | 885.4,904.6 | 882.5,899.3,916.7 | 69.0 | 31.0 | 3.284% | 1.45% |
| 8.0at% | 885.3,903.8 | 882.7,898.5,914.6 | 68.2 | 31.8 | 5.356% | 2.498% |
| 10.0at% | 884.5,903.1 | 882.6,899.2,915.7 | 71.4 | 28.6 | 6.877% | 2.755% |
表2 XPS分析YVO4:Ce3+晶体中Ce3+/Ce4+相对含量
Table 2 Ce3+/Ce4+ relative content in YVO4:Ce3+ crystals
| Ce Concentration | Ce3+ Binding energy/eV | Ce4+ Binding energy/eV | Ce3+ Relative content/% | Ce4+ Relative content/% | Ce3+ Rela content/% | Ce4+ Rela content/% |
|---|---|---|---|---|---|---|
| 1.0at% | 891.1,906.8 | 916.3 | 64.8 | 35.2 | 0.613% | 0.333% |
| 2.0at% | 884.2,904.3 | 898.1,916.2 | 75.0 | 25.0 | 1.426% | 0.476% |
| 3.0at% | 886.3,904.8 | 898.1,898.3,915.6 | 65.4 | 34.6 | 1.896% | 1.003% |
| 5.0at% | 885.4,904.6 | 882.5,899.3,916.7 | 69.0 | 31.0 | 3.284% | 1.45% |
| 8.0at% | 885.3,903.8 | 882.7,898.5,914.6 | 68.2 | 31.8 | 5.356% | 2.498% |
| 10.0at% | 884.5,903.1 | 882.6,899.2,915.7 | 71.4 | 28.6 | 6.877% | 2.755% |
| Ce concentration | V4+ binding energy/eV | V5+ binding energy/eV | V4+ relative content/% | V5+ relative content/% |
|---|---|---|---|---|
| 1.0at% | 515.5 | 517 | 0.24 | 99.76 |
| 2.0at% | 515.5 | 517 | 0.32 | 99.68 |
| 3.0at% | 515.5 | 517 | 0.58 | 99.42 |
| 5.0at% | 515.5 | 517 | 0.8 | 99.2 |
| 8.0at% | 515.5 | 517 | 1.37 | 98.63 |
| 10.0at% | 515.5 | 517 | 1.62 | 98.38 |
表3 XPS分析YVO4:Ce3+晶体中V4+/V5+相对含量
Table 3 V4+/V5+ relative content in YVO4:Ce3+ crystals
| Ce concentration | V4+ binding energy/eV | V5+ binding energy/eV | V4+ relative content/% | V5+ relative content/% |
|---|---|---|---|---|
| 1.0at% | 515.5 | 517 | 0.24 | 99.76 |
| 2.0at% | 515.5 | 517 | 0.32 | 99.68 |
| 3.0at% | 515.5 | 517 | 0.58 | 99.42 |
| 5.0at% | 515.5 | 517 | 0.8 | 99.2 |
| 8.0at% | 515.5 | 517 | 1.37 | 98.63 |
| 10.0at% | 515.5 | 517 | 1.62 | 98.38 |
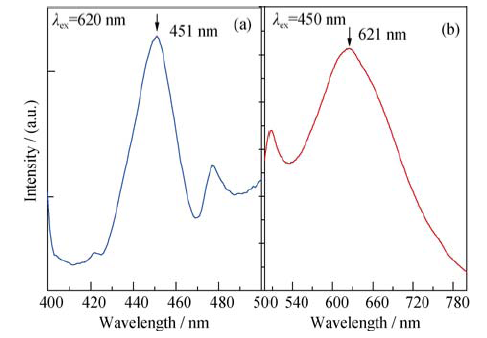
图10 YVO4:Ce3+晶体在620 nm激发下的上转换发光和450 nm激发下的红光发射
Fig. 10 Up-conversion luminescence (λex = 620 nm ) and red emission (λex = 450 nm ) of YVO4:Ce3+ crystal
| [1] | LI P L, WANG Y, GUO Q L.Research progress in single host white light emitting phosphor for white LEDs.Chinese Sci. Bull., 2011, 56(7): 488-503. |
| [2] | LI P L, WANG Z D, LUO Z Y, et al.Progress of phosphors for UV and near-UV based white LEDs. Journal of Synthetic Crystals, 2015, 44(11): 2954-2962. |
| [3] | ZHANG S H, ZHOU M B, HU J F, et al.Research progress in preparation of single phase silicate phosphor for nuv-white light emitting diodes.Materials Review, 2009, 23(5): 25-29. |
| [4] | WANG Z J, TIAN Z, YOU J Q, et al.Recent development on single-phase white emitting phosphors for white LEDs.Journal of the Chinese Ceramic Society, 2016, 44(1): 172-180. |
| [5] | RAUT S K, DHOBLE N S, DHOBLE S J.Optical properties of Eu, Dy, Mn activated M2SiO4,(M2=Ca, Sr, Zn) orthosilicate phosphors.Journal of Luminescence, 2013, 134: 325-332. |
| [6] | P S THAKER, S C GEDAM, S J DHOBLE, et al.Luminescence of KCaSO4Cl: X, Y (X=Eu or Ce; Y=Dy or Mn) halosulfate material.Journal of Luminescence, 2011, 131: 1612-1616. |
| [7] | RIWOTZKI K, HAASE M.Wet-chemical synthesis of doped colloidal nanoparticles: YVO4: Ln (Ln=Eu, Sm, Dy). The Journal of Physical Chemistry B, 1998, 102(50): 10129-10135. |
| [8] | SHEN L J, LI B, WANG Z Z, et al.Research development of rare earth vanadate liuminesence materials.Chinese Rare Earhts, 2015, 36(6): 129-137. |
| [9] | WITOLD R R, TOMASZ N, JAROS L K.Luminescence and energy transfer phenomena in YVO4 single crystal co-doped with Tm3+ and Eu3+.Journal of Luminescence, 2015, 162: 134-139. |
| [10] | BLASSE G.On the Eu3+ fluorescence of mixed metal oxides. IV. The photoluminescent efficiency of Eu3+-activated oxides.The Journal of Chemical Physics, 1966, 45(7): 2356-2360. |
| [11] | SHEN L J, LI B, WANG Z Z, et al.Vacuum ultraviolet spectra of YVO4:Tm3+.Chinese Journal of Luminescence, 2014, 35(9): 1034-1039. |
| [12] | DEVI C V, SINGH N R.Effect of annealing on the luminescence properties of YVO4: Dy phosphor on co-doping Pb2+ ions.Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2015, 146: 331-341. |
| [13] | CHEN X B, ZHOU G, ZHOU Y F, et al.Near-Infrared quantum cutting downconversion luminescence of Yb3+ ion cooperative energy transferred from YVO4 matrix donor. Spectroscopy and Spectral Analysis, 2015, 35(2): 315-319. |
| [14] | ZHANG S C, GU J X, JIA G Z, et al.Effect of annealing on the spectroscopy performance of YVO4: Ce3+ single crystal.Optical Materials, 2015, 39: 178-181. |
| [15] | ZHANG S C, RUAN R F, JIA G Z, et al.Blue-emitting properties of Ce3+ doped YVO4 under ultraviolet excitation.Journal of Inorganic Materials, 2014, 29(10): 1067-1072. |
| [16] | PAN Y X, WANG W, LIU G K, et al.Correlation between structure variation and luminescence red shift in YAG:Ce.Journal of Alloys and Compounds, 2009, 488: 638-642. |
| [17] | XIN J, YU Y C, LIU J J, et al.Synthesis of fluorescent YVO4: Eu nano-particle and its application in developing fingerprint.Chemical Research, 2010, 21(2): 1-6. |
| [18] | WANG Y F, WANG S, RUAN Y F, et al.Photoluminescence properties of Ce and Eu co-doped YVO4 crystals.Journal of Alloys and Compounds, 2013, 551: 262-266. |
| [19] | REN G H, PEI Y, WU Y T, et al. Influence of Ce doping concentration on the luminescence properties of LaCl3:Ce scintillation crystals. Acta Physica Sinica, 2014, 63(3): 037802-1-6. |
| [20] | WANG D J.Theoretical Calculation of Electronic Structures and 4f→5d Transitions of Lanthanide Ions Doped in Crystals. Anhui: University of Science and Technology of China A Dissertation for Doctor's Degree, 2009. |
| [21] | NINGTHOUJAM R S, SINGH L R, SUDARSAN V, et al.Energy transfer process and optimum emission studies in luminescence of core-shell nanoparticles: YVO4:Eu-YVO4 and surface state analysis.Journal of Alloys and Compounds, 2009, 484: 782-789. |
| [22] | P DORENBOS.5d-level energies of Ce3+ and the crystalline environment. IV. Aluminates and “simple” oxides.Journal of Luminescence, 2002, 99: 283-299. |
| [23] | DENG T G, XIA Z G, DING H.Effect of [PO4]3-/[VO4]3- substitution on the structure and luminescence properties of Ca5[(P,V)O4]]3F: Eu3+ phosphors.Chemical Physics Letters, 2015, 637: 67-70. |
| [24] | BLASSE G, A BRIL. Luminescence of phosphors based on host lattices ABO4 (A is Sc, In; B is P, V, Nb).The Journal of Chemical Physics, 1969, 50(7): 2974-2980. |
| [25] | SVITASHEVA S N, GILINSK A M.Influence of doping level on shift of the absorption edge of gallium nitride films (Burstein-Moss effect).Applied Surface Science, 2013, 281: 109-112. |
| [26] | DORENBOS P.The 5d level positions of the trivalent lanthanides in inorganic compounds.Journal of Luminescence, 2000, 91: 155-176. |
| [27] | ANANDAN C, BERA P.XPS studies on the interaction of CeO2 with silicon in magnetronsputtered CeO2 thin films on Si and Si3N4 substrates.Applied Surface Science, 2013, 283: 297-303. |
| [28] | HEIKKINEN H, JOHANSSON L S, NYKANEN E, et al.An XPS study of SrS:Ce thin films for electroluminescent devices.Applied Surface Science, 1998, 133: 205-212. |
| [29] | ZOU Y Q, LI X J, XU J, et al.Different chemical valence of V element in the YVO4 crystal.Journal of Synthetic Crystals, 2003, 32(1): 27-30. |
| [30] | WANG Y F, WU Z L, RUAN Y F, et al. Spectroscopic properties of cerium doped YVO4 crystals and analysis on valence state of cerium ion. Acta Physica Sinica, 2012, 61(22): 228105(1)- 228105(8). |
| [31] | YANG Z Y, WEI Q, HAO Y.Investigations of lattice distortion and EPR parameters for YAG:V2+ laser crystal.Journal of Synthetic Crystals, 2005, 34(3): 491-495. |
| [32] | HUANG Y P.Theoretical studies of optical spectra and EPR parameters for V4+ in ThSiO4 crystal.Journal of Synthetic Crystals, 2008, 37(5): 1145-1147. |
| [33] | GREGORIO S D, GREENBLATT M, PIFER J H, et al.An ESR and optical study of V4+ in zircon-type crystals.The Journal of Chemical Physics, 1982, 76(6): 2931-2937. |
| [34] | TOMASZ G, ARTUR T.Up-conversion luminescence of GdOF: Yb3+, Ln3+ (Ln=Ho, Tm, Er) nanocrystals.Journal of Alloys and Compounds, 2016, 660: 235-243. |
| [1] | 潘雨舟, 何法鉴, 徐路路, 戴世勋. 980 nm LD泵浦下Dy3+/Yb3+共掺碲酸盐玻璃3 μm波段中红外宽带发光特性[J]. 无机材料学报, 2025, 40(5): 521-528. |
| [2] | 于嫚, 高荣耀, 秦玉军, 艾希成. 上转换发光纳米材料对钙钛矿太阳能电池迟滞效应和离子迁移动力学的影响[J]. 无机材料学报, 2024, 39(4): 359-366. |
| [3] | 彭跃红,任韦舟,邱建备,韩缙,杨正文,宋志国. 1550 nm激发层状BiOCl:Er3+上转换发光及温度传感特性[J]. 无机材料学报, 2020, 35(8): 902-908. |
| [4] | 张志洁,黄海瑞,程昆,郭少柯. 高效碳量子点/BiOCl纳米复合材料用于光催化污染物降解[J]. 无机材料学报, 2020, 35(4): 491-496. |
| [5] | 吕喜庆, 张环宇, 李瑞, 张梅, 郭敏. Nb2O5包覆对TiO2纳米阵列/上转换发光复合结构柔性染料敏化太阳能电池性能的影响[J]. 无机材料学报, 2019, 34(6): 590-598. |
| [6] | 高东, 张煜亮, 孙静, 范宏筠. 一步法合成特异性pH响应碳量子点及其发光机理研究[J]. 无机材料学报, 2019, 34(12): 1309-1315. |
| [7] | 史忠祥, 王晶, 关昕. Er3+掺杂调控NaY(WO4)2: Dy3+的上转换发光性能[J]. 无机材料学报, 2018, 33(5): 521-527. |
| [8] | 李永进, 刘群, 周玉婷, 邱建备, 宋志国. Yb3+-Tm3+共掺BiOBr纳米晶的近红外上转换发光特性研究[J]. 无机材料学报, 2016, 31(3): 279-284. |
| [9] | 周佳佳, 邱建荣. 单个纳米颗粒的上转换光谱现象研究[J]. 无机材料学报, 2016, 31(10): 1023-1030. |
| [10] | 朱美娟, 余建定, 张明辉, 谷彦静, 李 勤, 方必军, 赵洪阳, 沈 清. 无容器制备Er3+/Yb3+共掺La2O3-TiO2-ZrO2玻璃及其上转换发光研究[J]. 无机材料学报, 2015, 30(4): 391-396. |
| [11] | 彭 霞, 李淑星, 刘学建,黄毅华, 黄政仁, 李会利. Eu2+/Tb3+掺杂的Sr2Si5N8基荧光粉的制备与发光性能[J]. 无机材料学报, 2015, 30(4): 397-400. |
| [12] | 张继红, 陶海征, 顾少轩, 张高科, 刘 超, 赵修建. 面向太阳光全光谱应用铒掺杂硫卤玻璃上转换发光特性研究[J]. 无机材料学报, 2015, 30(3): 245-248. |
| [13] | 向军涛, 杜 鹏, 罗来慧, 方义权, 赵学洋, 胡旭波, 陈红兵. Er3+掺杂弛豫铁电材料PMNT的单晶生长与性能表征[J]. 无机材料学报, 2015, 30(2): 135-140. |
| [14] | 耿志明, 李 坤, 施东良, 施瑕玉, 黄海涛, 陈王丽华. 铌酸钾钠基透明上转换陶瓷材料制备及性能[J]. 无机材料学报, 2014, 29(12): 1265-1269. |
| [15] | 骆家亮, 吴云涛, 任国浩. Ce3+, Eu3+共掺Lu3Al5O12多晶的发光特性及能量传递研究[J]. 无机材料学报, 2014, 29(11): 1211-1217. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||