无机材料学报 ›› 2021, Vol. 36 ›› Issue (12): 1283-1289.DOI: 10.15541/jim20210100 CSTR: 32189.14.10.15541/jim20210100
所属专题: 【虚拟专辑】化学反应催化剂(2020~2021)
刘雯雯1( ), 苗雨欣1,2(
), 苗雨欣1,2( ), 张轶扉1, 王心宇1, 兰玉婷1, 赵震1,2(
), 张轶扉1, 王心宇1, 兰玉婷1, 赵震1,2( )
)
收稿日期:2021-02-18
修回日期:2021-05-13
出版日期:2021-12-20
网络出版日期:2021-05-25
通讯作者:
苗雨欣, 讲师. E-mail: yuxinmiao@synu.edu.cn;赵 震, 教授. E-mail: zhaozhen@synu.edu.cn;zhenzhao@cup.edu.cn
作者简介:刘雯雯(1997-), 女, 硕士研究生. E-mail: 1753305303@qq.com
基金资助:
LIU Wenwen1( ), MIAO Yuxin1,2(
), MIAO Yuxin1,2( ), ZHANG Yifei1, WANG Xinyu1, LAN Yuting1, ZHAO Zhen1,2(
), ZHANG Yifei1, WANG Xinyu1, LAN Yuting1, ZHAO Zhen1,2( )
)
Received:2021-02-18
Revised:2021-05-13
Published:2021-12-20
Online:2021-05-25
Contact:
MIAO Yuxin, lecturer. E-mail: yuxinmiao@synu.edu.cn;ZHAO Zhen, professor. E-mail: zhaozhen@synu.edu.cn;zhenzhao@cup.edu.cn
About author:LIU Wenwen(1997-), female, Master candidate. E-mail: 1753305303@qq.com
Supported by:摘要:
如何制备性能高效、稳定的纳米Pt催化剂, 对提高肉桂醛C=O加氢选择性具有重要的实际价值和科学意义。本研究采用共沉淀法和水热法制备不同形貌的MgAl水滑石(LDH), 用等体积浸渍法制备负载型Pt/LDH催化剂, 研究不同形貌的LDH对Pt催化肉桂醛选择加氢性能的影响。利用不同表征方法对催化剂结构、形貌、表面理化性能进行分析。结果表明, LDH载体的形貌对Pt/LDH催化剂的结构和性能影响较大, 具有片层结构的LDH纳米片与活性组分Pt之间的相互作用易于贵金属Pt的分散和稳定。同时, LDH载体中存在丰富的碱性位点和表面羟基, 有利于提高催化加氢活性和稳定性。催化性能评价表明, 在反应温度为40 ℃、反应压力为1 MPa、反应时间为120 min的条件下, 肉桂醇(CMO)的选择性为82.1%, 肉桂醛的转化率为79.8%, 经过5轮循环测试, 催化剂仍具有较好的稳定性。
中图分类号:
刘雯雯, 苗雨欣, 张轶扉, 王心宇, 兰玉婷, 赵震. 不同形貌MgAl LDH的制备及Pt/LDH的催化加氢性能[J]. 无机材料学报, 2021, 36(12): 1283-1289.
LIU Wenwen, MIAO Yuxin, ZHANG Yifei, WANG Xinyu, LAN Yuting, ZHAO Zhen. Preparation of MgAl LDH with Various Morphologies and Catalytic Hydrogenation Performance of Pt/LDH Catalysts[J]. Journal of Inorganic Materials, 2021, 36(12): 1283-1289.
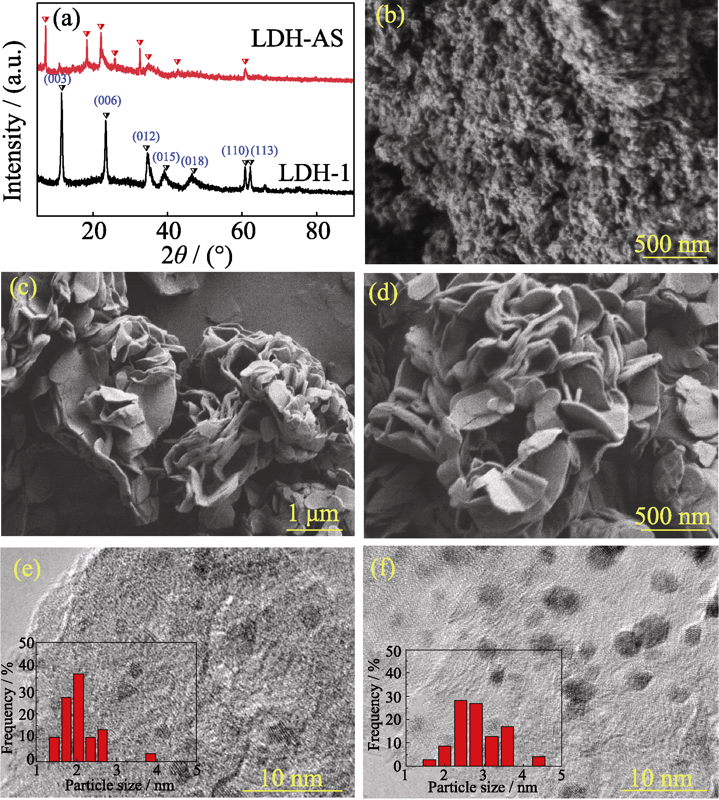
图1 不同形貌LDH的XRD图谱(a), LDH-1(b)和LDH-AS (c, d)的SEM照片, Pt/LDH-1(e)和Pt/LDH-AS(f)的TEM照片
Fig. 1 XRD patterns of different morphologies LDH (a), SEM images of LDH-1 (b) and LDH-AS (c, d), TEM images of Pt/LDH-1 (e) and Pt/LDH-AS (f)
| Catalyst | Conversion/% | Selectivity/% | ||
|---|---|---|---|---|
| CMO | HCMA | HCMO | ||
| Pt/LDH-1 | 79.8 | 82.1 | 12.6 | 5.3 |
| Pt/LDH-AS | 42.3 | 89.4 | 8.5 | 2.1 |
表1 不同形貌Pt催化剂对肉桂醛加氢性能影响
Table 1 Effects of Pt catalysts with various morphologies on the hydrogenation properties of cinnamaldehyde
| Catalyst | Conversion/% | Selectivity/% | ||
|---|---|---|---|---|
| CMO | HCMA | HCMO | ||
| Pt/LDH-1 | 79.8 | 82.1 | 12.6 | 5.3 |
| Pt/LDH-AS | 42.3 | 89.4 | 8.5 | 2.1 |
| Catalyst | Pt content/ at% | Ptδ+/Pt0 | Oads/(Oads+Olat) | Mg/Al atomic ratio |
|---|---|---|---|---|
| Pt/LDH-1 | 0.35 | 2.51 | 0.35 | 1.19 |
| Pt/LDH-AS | 0.28 | 2.30 | 0.21 | 0.98 |
表2 XPS测定Pt/LDH-1和Pt/LDH-AS催化剂的表面元素组成
Table 2 Surface elemental compositions of Pt/LDH-1 and Pt/LDH-AS catalysts determined by XPS
| Catalyst | Pt content/ at% | Ptδ+/Pt0 | Oads/(Oads+Olat) | Mg/Al atomic ratio |
|---|---|---|---|---|
| Pt/LDH-1 | 0.35 | 2.51 | 0.35 | 1.19 |
| Pt/LDH-AS | 0.28 | 2.30 | 0.21 | 0.98 |
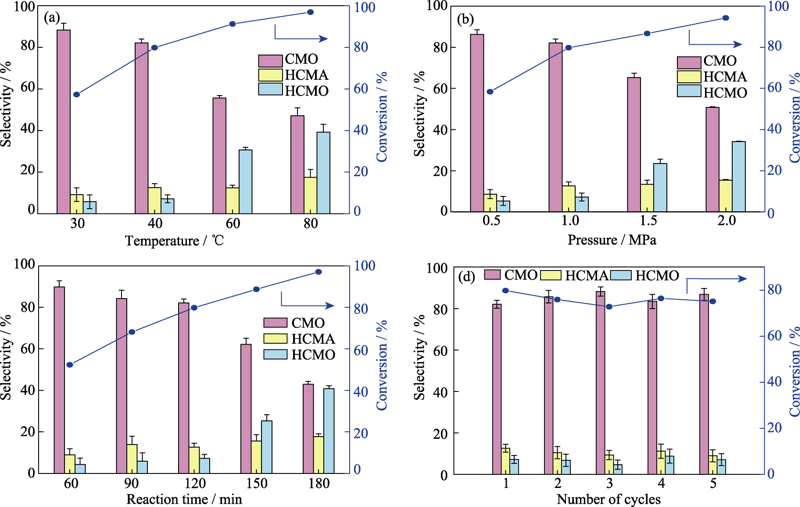
图3 温度(a), 压力(b), 反应时间(c)和循环稳定性(d)对Pt/LDH-1催化剂的催化性能的影响
Fig. 3 Effect of temperature (a), pressure (b), reaction time (c), and recycling stability (d) on catalytic performances of Pt/LDH-1 catalysts Curves represent the conversion rate, while bar charts represent the selectivity
| [1] |
XU Z, DUONG-VIET C, LIU Y, et al. Macroscopic graphite felt containing palladium catalyst for liquid-phase hydrogenation of cinnamaldehyde. Applied Catalysis B: Environmental, 2019, 244: 128-139.
DOI URL |
| [2] |
CAO Z, BU J, ZHONG Z, et al. Selective hydrogenation of cinnamaldehyde to cinnamyl alcohol over BN supported Pt catalysts at room temperature. Applied Catalysis A: General, 2019, 578: 105-115.
DOI URL |
| [3] |
TAYLOR M J, DURNDELL L J, ISAACS M A, et al. Highly selective hydrogenation of furfural over supported Pt nanoparticles under mild conditions. Applied Catalysis B: Environmental, 2016, 180: 580-585.
DOI URL |
| [4] |
XIN H, XUE Y, ZHANG W, et al. CoxFe1-xAl2O4+δ composite oxides supported Pt nanoparticles as efficient and recyclable catalysts for the liquid-phase selective hydrogenation of cinnamaldehyde. Journal of Catalysis, 2019, 380: 254-266.
DOI URL |
| [5] |
TAN Y, LIU X Y, ZHANG L, et al. ZnAl-hydrotalcite-supported Au25 nanoclusters as precatalysts for chemoselective hydrogenation of 3-nitrostyrene. Angewandte Chemie International Edition, 2017, 56: 2709-2713.
DOI URL |
| [6] |
REN W, DING T, YANG Y, et al. Identifying oxygen activation/ oxidation sites for efficient soot combustion over silver catalysts interacted with nanoflower-like hydrotalcite-derived CoAlO metal oxides. ACS Catalysis, 2019, 9(9): 8772-8784.
DOI URL |
| [7] |
YANG Y, RAO D, CHEN Y, et al. Selective hydrogenation of cinnamaldehyde over Co-based intermetallic compounds derived from layered double hydroxides. ACS Catalysis, 2018, 8(12): 11749-11760.
DOI URL |
| [8] |
GAO X, DAI H, PENG L, et al. Effect of hydrotalcites interlayer water on Pt-catalyzed aqueous-phase selective hydrogenation of cinnamaldehyde. ACS Applied Materials Interfaces, 2020, 12: 2516-2524.
DOI URL |
| [9] |
ZHU Y, AN Z, HE J. Single-atom and small-cluster Pt induced by Sn (IV) sites confined in an LDH lattice for catalytic reforming. Journal of Catalysis, 2016, 341: 44-54.
DOI URL |
| [10] |
CAI T, ZHANG P, SHEN X, et al. Synthesis of Pt-loaded NiFe- LDH nanosheets on wood veneer for efficient gaseous formaldehyde degradation. ACS Applied Materials Interfaces, 2020, 12: 37147-37154.
DOI URL |
| [11] |
HAN J, MENG X, LU L, et al. Triboelectric nanogenerators powered electrodepositing tri-functional electrocatalysts for water splitting and rechargeable zinc-air battery: a case of Pt nanoclusters on NiFe-LDH nanosheets. Nano Energy, 2020, 72: 104669.
DOI URL |
| [12] |
ANBARASAN R, LEE W D, IM S S. Adsorption and intercalation of anionic surfactants onto layered double hydroxides-XRD study. Bulletin of Materials Science, 2005, 28(2): 145-149.
DOI URL |
| [13] | DENG L, ZENG H X, SHI Z, et al. Sodium dodecyl sulfate intercalated and acrylamide anchored layered double hydroxides: a multifunctional adsorbent for highly efficient removal of Congo red. Journal of Colloid & Interface Science, 2018, 521: 172-182. |
| [14] |
ZHAO H, NAGY K L. Dodecyl sulfate-hydrotalcite nanocomposites for trapping chlorinated organic pollutants in water. Journal of Colloid and Interface Science, 2004, 274(2): 613-624.
DOI URL |
| [15] |
SIDERIS P J, NIELSEN U G, GAN Z H, et al. Mg/Al ordering in layered double hydroxides revealed by multinuclear NMR spectroscopy. Science, 2008, 321(5885): 113-117.
DOI URL |
| [16] |
WANG X, MUJTABA J, FANG F, et al. Constructing aligned γ-Fe2O3 nanorods with internal void space anchored on reduced graphene oxide nanosheets for excellent lithium storage. RSC Advances, 2015, 5(111): 91574-91580.
DOI URL |
| [17] |
DONG Y L, ZHANG X F, CHENG X L, et al. Highly selective NO sensor at room temperature based on nanocomposites of hierarchical nanosphere-like α-FeO and reduced graphene oxide. RSC Advances, 2014, 4(101): 57493-57500.
DOI URL |
| [18] | TANG W, DENG Y, LI W, et al. Importance of porous structure and synergistic effect on the catalytic oxidation activities over hierarchical Mn-Ni composite oxides. Catalysis Science & Technology, 2016, 6(6): 1710-1718. |
| [19] |
CHEN X, WANG P, FANG P, et al. Design strategies for SCR catalysts with improved N2 selectivity: the significance of nano- confining effects by titanate nanotubes. Environmental Science Nano, 2017, 4(2): 437-447.
DOI URL |
| [20] |
LIU Z T, WANG C X, LIU Z W, et al. Selective hydrogenation of cinnamaldehyde over Pt-supported multi-walled carbon nanotubes: Insights into the tube-size effects. Applied Catalysis A, General, 2008, 344(1): 114-123.
DOI URL |
| [21] |
BERA P, PRIOLKAR K R, GAYEN A, et al. Ionic dispersion of Pt over CeO2 by the combustion method: structural investigation by XRD, TEM, XPS, and EXAFS. Chemistry of Materials, 2003, 15(10): 2049-2060.
DOI URL |
| [22] |
STASSI J P, ZGOLICZ P D, DE MIGUEL S R, et al. Formation of different promoted metallic phases in PtFe and PtSn catalysts supported on carbonaceous materials used for selective hydrogenation. Journal of Catalysis, 2013, 306: 11-29.
DOI URL |
| [23] |
PAN H, LI J, LU J, et al. Selective hydrogenation of cinnamaldehyde with PtFex/Al2O3@SBA-15 catalyst: enhancement in activity and selectivity to unsaturated alcohol by Pt-FeOx and Pt-Al2O3@SBA-15 interaction. Journal of Catalysis, 2017, 354: 24-36.
DOI URL |
| [24] |
ZHANG S, FAN G, LI F. Lewis-base-promoted copper-based catalyst for highly efficient hydrogenation of dimethyl 1,4- cyclohexane dicarboxylate. Green Chemistry, 2013, 15(9): 2389-2393.
DOI URL |
| [1] | 荀道祥, 罗序维, 周明冉, 何佳乐, 冉茂进, 胡执一, 李昱. 锂硒电池ZIF-L衍生氮掺杂碳纳米片/碳布自支撑电极的电化学性能研究[J]. 无机材料学报, 2024, 39(9): 1013-1021. |
| [2] | 施桐, 甘乔炜, 刘东, 张莹, 冯浩, 李强. 自支撑Bi@Cu纳米树电极高效电化学还原CO2制甲酸[J]. 无机材料学报, 2024, 39(7): 810-818. |
| [3] | 杨恩东, 李宝乐, 张珂, 谭鲁, 娄永兵. ZnCo2O4-ZnO@C@CoS核壳复合材料的制备及其在超级电容器中的应用[J]. 无机材料学报, 2024, 39(5): 485-493. |
| [4] | 冯星哲, 马董云, 王金敏. 多孔NiMn-LDH纳米片薄膜的溶剂热生长及其电致变色性能[J]. 无机材料学报, 2024, 39(12): 1391-1396. |
| [5] | 王新玲, 周娜, 田亚文, 周明冉, 韩静茹, 申远升, 胡执一, 李昱. SnS2/ZIF-8衍生二维多孔氮掺杂碳纳米片复合材料的锂硫电池性能研究[J]. 无机材料学报, 2023, 38(8): 938-946. |
| [6] | 牛海滨, 黄佳慧, 李倩文, 马董云, 王金敏. 多孔NiMoO4纳米片薄膜的直接水热生长及其电致变色性能[J]. 无机材料学报, 2023, 38(12): 1427-1433. |
| [7] | 王如意, 徐国良, 杨蕾, 邓崇海, 储德林, 张苗, 孙兆奇. p-n异质结BiVO4/g-C3N4光阳极的制备及其光电化学水解性能[J]. 无机材料学报, 2023, 38(1): 87-96. |
| [8] | 李鹏鹏, 王兵, 王应德. 基于火焰退火多孔CeO2纳米片的环境监测用超快CO气体传感器[J]. 无机材料学报, 2021, 36(11): 1223-1230. |
| [9] | 舒孟洋, 陆嘉琳, 张志洁, 沈涛, 徐家跃. CsPbBr3钙钛矿量子点/C3N4超薄纳米片0D/2D复合材料: 增强的稳定性和光催化活性[J]. 无机材料学报, 2021, 36(11): 1217-1222. |
| [10] | 杨劢, 朱敏, 陈雨, 朱钰方. FePS3纳米片制备及其体外光热-光动力学联合治疗性能研究[J]. 无机材料学报, 2021, 36(10): 1074-1082. |
| [11] | 谢雪, 吴建荣, 蔡晓军, 郝俊年, 郑元义. 光热/pH响应B-CuS-DOX纳米药物用于化疗-光热协同治疗肿瘤[J]. 无机材料学报, 2021, 36(1): 81-87. |
| [12] | 赵宇鹏,贺勇,张敏,史俊杰. 新型二维Zr2CO2/InS异质结可见光催化产氢性能的第一性原理研究[J]. 无机材料学报, 2020, 35(9): 993-998. |
| [13] | 李锐,王浩,付强,田子玉,王建旭,马小健,杨剑,钱逸泰. 构建亲锂铜基3D集流体实现金属锂的均匀沉积[J]. 无机材料学报, 2020, 35(8): 882-888. |
| [14] | 董丽佳, 郭筱洁, 李雪, 陈朝贵, 金阳, AHMED Alsaedi, TASAWAr Hayat, 赵轻舟, 盛国栋. 不同pH条件下硫化钼纳米片吸附Cd(II)的微观机制研究[J]. 无机材料学报, 2020, 35(3): 293-300. |
| [15] | 魏鑫, 卢占会, 王路平, 方明. 可见光下Bi2WO6纳米片高效光降解四环素的机理研究[J]. 无机材料学报, 2020, 35(3): 324-328. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||