无机材料学报 ›› 2022, Vol. 37 ›› Issue (11): 1170-1180.DOI: 10.15541/jim20220158 CSTR: 32189.14.10.15541/jim20220158
黄田1,2,3( ), 赵运超1,2,3, 李琳琳2,3,4(
), 赵运超1,2,3, 李琳琳2,3,4( )
)
收稿日期:2022-03-21
修回日期:2022-04-24
出版日期:2022-11-20
网络出版日期:2022-06-16
通讯作者:
李琳琳, 研究员. E-mail: lilinlin@binn.cas.cn作者简介:黄田(1996-), 女, 硕士研究生. E-mail: huangtian@binn.cas.cn
基金资助:
HUANG Tian1,2,3( ), ZHAO Yunchao1,2,3, LI Linlin2,3,4(
), ZHAO Yunchao1,2,3, LI Linlin2,3,4( )
)
Received:2022-03-21
Revised:2022-04-24
Published:2022-11-20
Online:2022-06-16
Contact:
LI Linlin, professor. E-mail: lilinlin@binn.cas.cnAbout author:HUANG Tian (1996-), female, Master candidate. E-mail: huangtian@binn.cas.cn
Supported by:摘要:
随着纳米医学的发展, 利用纳米材料在外源超声波的刺激下催化产生过量的活性氧物种(Reactive Oxygen Species, ROS)以治疗疾病的方法, 被称为声动力疗法(Sonodynamic Therapy, SDT), 已引起人们的广泛关注。目前, 开发可用于SDT的高效声敏剂用于提高ROS产率, 仍然是当前研究和未来临床转化的最大挑战之一。近年来, 得益于压电电子学和压电光电子学的兴起, 基于压电半导体纳米材料的新型声敏剂在SDT中崭露头角, 显示出良好的应用前景。本文从压电半导体的结构出发, 介绍了压电半导体纳米材料应用于SDT的机理研究, 以及利用压电半导体纳米材料作为声敏剂在声动力学癌症治疗及相关抗菌性能方面所取得的研究进展。最后, 本文对该领域存在的问题以及未来的发展趋势进行了展望。
中图分类号:
黄田, 赵运超, 李琳琳. 压电半导体纳米材料在声动力疗法中的应用进展[J]. 无机材料学报, 2022, 37(11): 1170-1180.
HUANG Tian, ZHAO Yunchao, LI Linlin. Piezoelectric Semiconductor Nanomaterials in Sonodynamic Therapy: a Review[J]. Journal of Inorganic Materials, 2022, 37(11): 1170-1180.
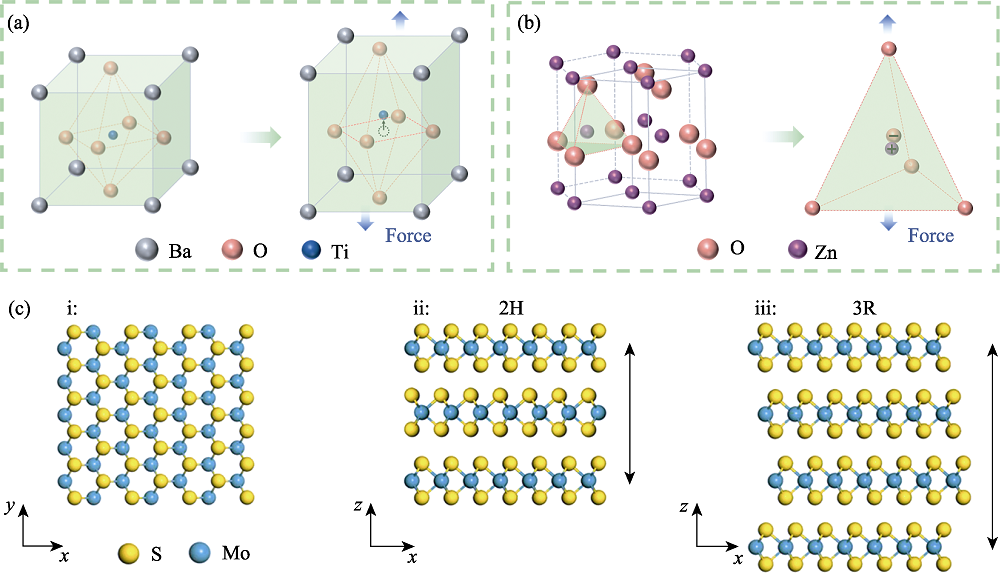
图1 压电半导体的结构特点[24]
Fig. 1 Structural characteristics of piezoelectric semiconductors[24] (a,b) Crystal structures of (a) perovskite BaTiO3 and (b) wurtzite ZnO; (c) Influence of stacking on the piezoelectric effect of MoS2 at crystal structure of (i) single-layer, (ii) 2H and (iii) 3R
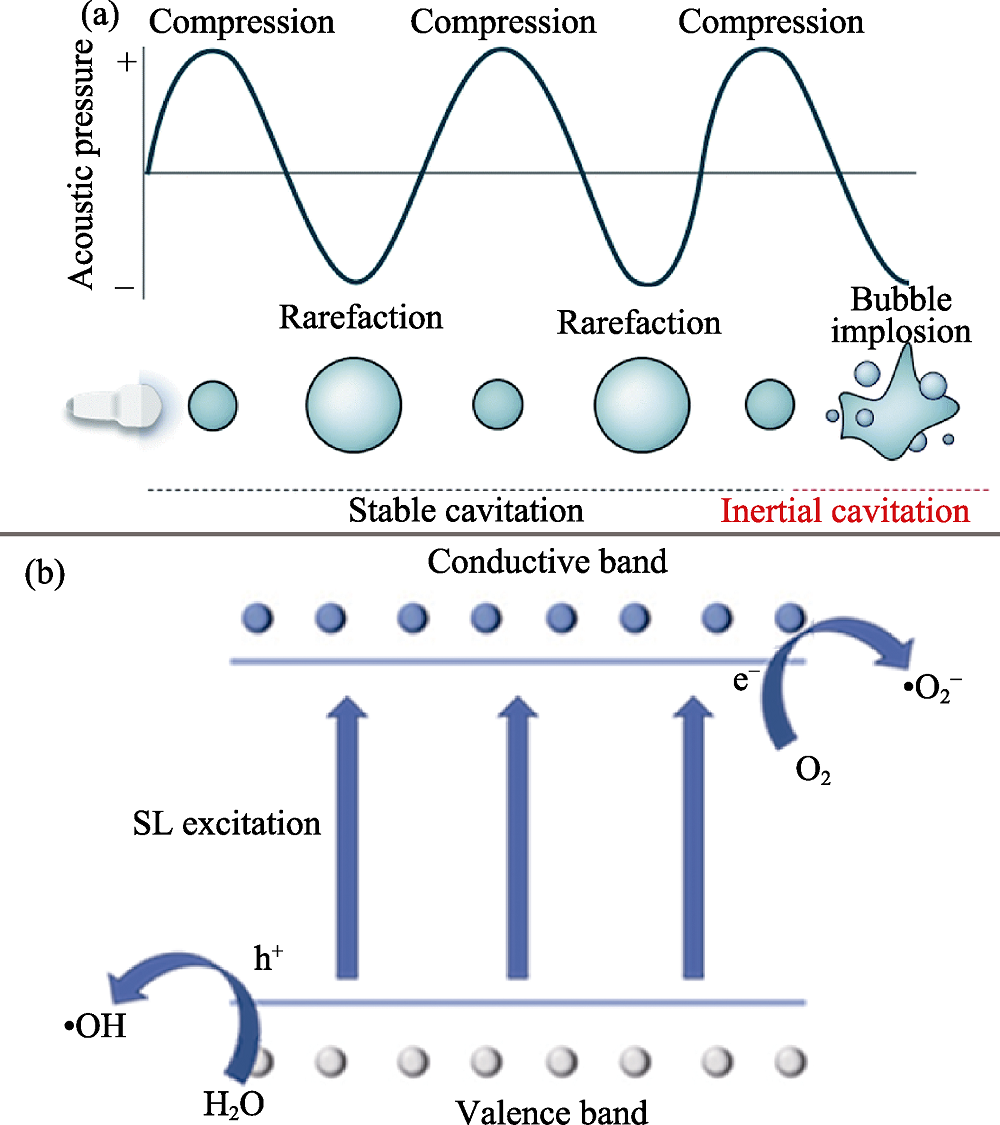
图2 空化效应与声致发光[25]
Fig. 2 Cavitation effect and sonoluminescence (SL)[25] (a) Schematic of cavitation effect with ultrasound; (b) Illustration of SL-excited photocatalysis
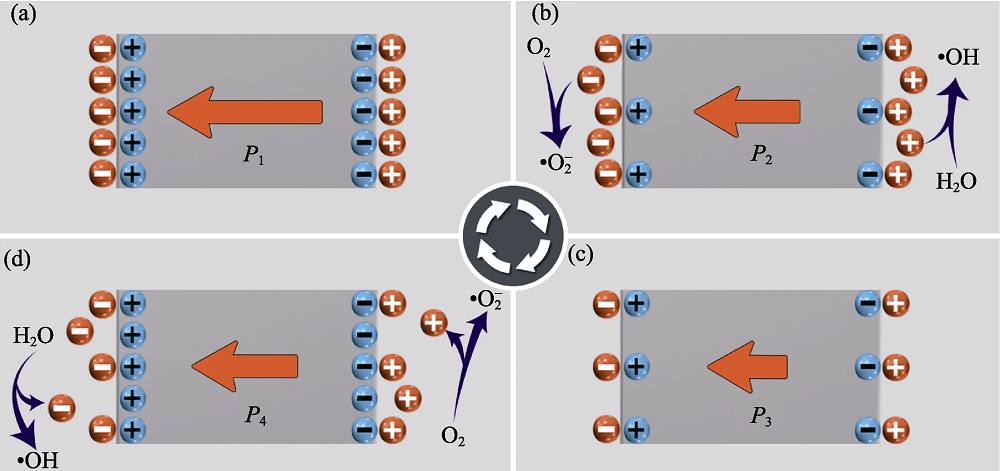
图3 压电催化示意图[32]
Fig. 3 Schematic of piezocatalysis[32] (a) Original electrostatic balance state of a poled piezoelectric material; (b) Charge release and ROS production under stress; (c) Modified electrostatic balance state under maximum stress; (d) Adsorption of charges from the surrounding electrolyte under reduced stress, and the opposite charges in the electrolyte are involved in ROS production
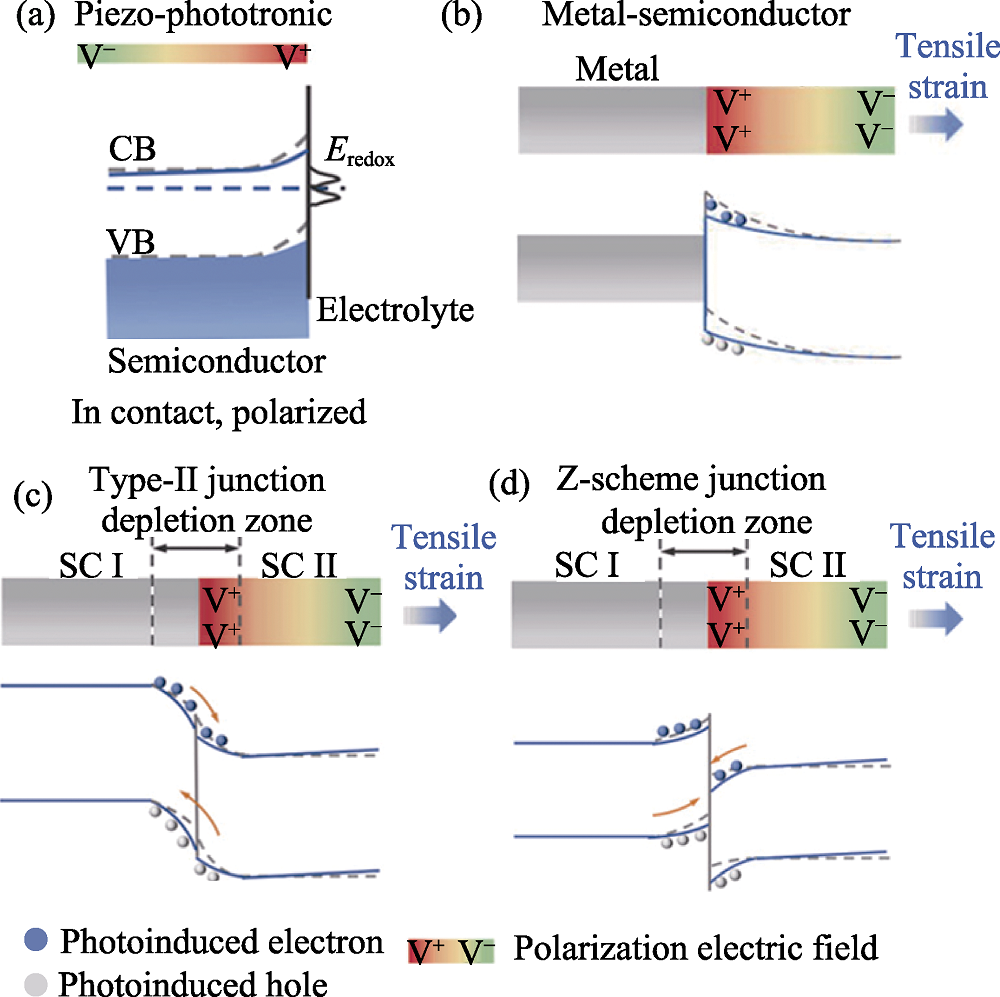
图4 压电光电子学效应对载流子迁移的影响[34]
Fig. 4 Influence of piezo-phototronic effect on carrier migration[34] (a) Semiconductor-electrolyte; (b) Metal-semiconductor; (c) Type-II; (d) Z-scheme CB: Conduction band; VB: Valence band; SC: Semiconductor
| Application | Nanomaterial | Frequency/MHz | Power/(W·cm-2) | Duty ratio/% | Duration/min | Ref. |
|---|---|---|---|---|---|---|
| Cancer treatment | BP | 1 | 1.5 | - | 10 (4 times) | [ |
| T-BTO | 1 | 1.0 | 50 | 10 (3 times) | [ | |
| Bi2MoO6 | 0.04 | 3.0 | 50 | 5 (3 times) | [ | |
| Au@BP | 1 | 2.0 | 40 | 2.5 (4 times) | [ | |
| D-ZnOx:Gd | 1 | 1.0 | 50 | - | [ | |
| Antibacteria | HNTM-MoS2 | 1 | 1.5 | 50 | 15 (Twice) | [ |
| Au@BTO | 1 | 1.5 | 50 | 3 (Once) | [ |
表1 压电半导体纳米材料用于SDT的动物实验中所使用的超声激发装置的相关参数
Table 1 Parameters of ultrasonic excitation devices used in animal experiments of sonodynamic therapy with piezoelectric semiconductor nanomaterials
| Application | Nanomaterial | Frequency/MHz | Power/(W·cm-2) | Duty ratio/% | Duration/min | Ref. |
|---|---|---|---|---|---|---|
| Cancer treatment | BP | 1 | 1.5 | - | 10 (4 times) | [ |
| T-BTO | 1 | 1.0 | 50 | 10 (3 times) | [ | |
| Bi2MoO6 | 0.04 | 3.0 | 50 | 5 (3 times) | [ | |
| Au@BP | 1 | 2.0 | 40 | 2.5 (4 times) | [ | |
| D-ZnOx:Gd | 1 | 1.0 | 50 | - | [ | |
| Antibacteria | HNTM-MoS2 | 1 | 1.5 | 50 | 15 (Twice) | [ |
| Au@BTO | 1 | 1.5 | 50 | 3 (Once) | [ |
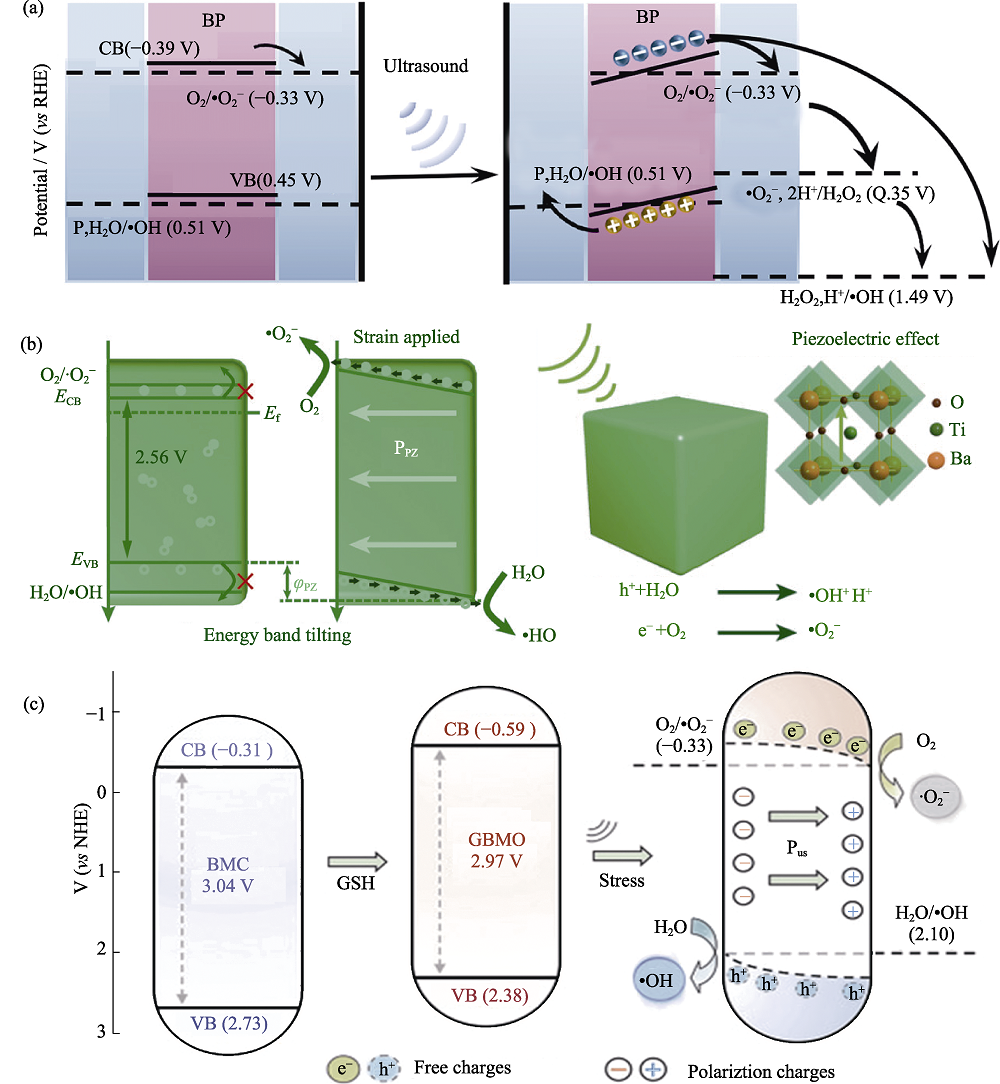
图5 压电半导体纳米材料超声条件下的能带倾斜增强SDT抗肿瘤应用[39⇓-41]
Fig. 5 Anti-tumor application of piezoelectric semiconductor nanomaterials in SDT enhanced by band tilt under ultrasound irradiation[39⇓-41] (a, b)Band structures of (a) black phosphorus (BP) nanosheets[39] and (b) T-BaTiO3 nanoparticles[40]; (c) Bi2MoO6 nanorods (BMO NRs) and GSH-activated BMO NRs (GBMO NRs) and their ROS generation under ultrasonic irradiation[41];CB: Conduction band; VB: Valence band; RHE: Relative hyedrogen electrode; NHE: Normal hydrogen electrode
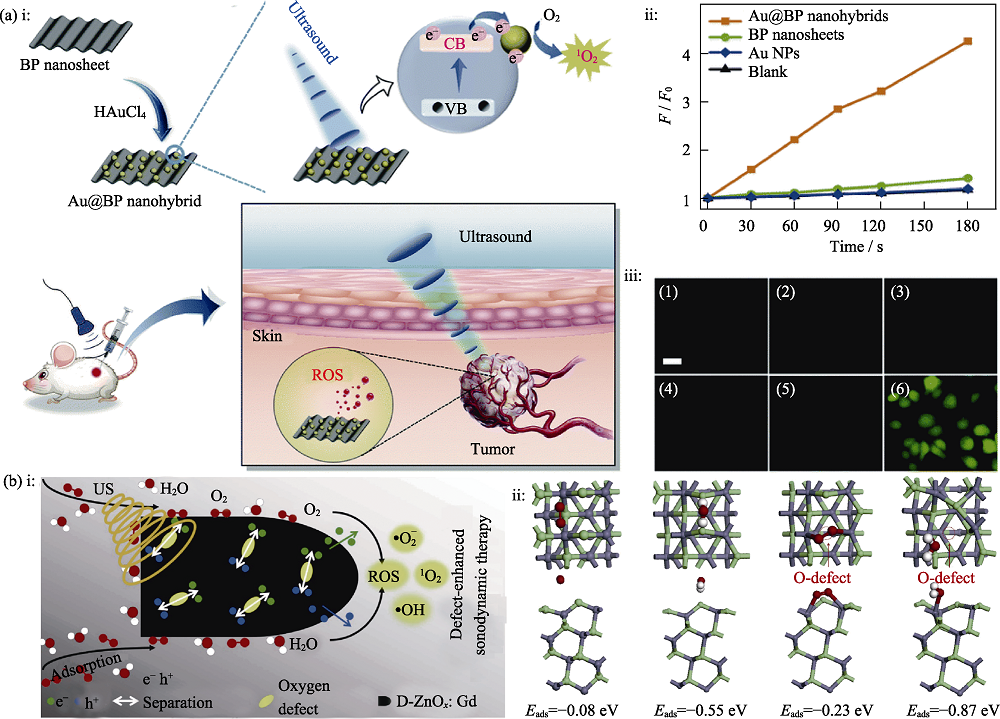
图6 在压电半导体纳米材料上构建异质结或引入缺陷来提高SDT效率[42-43]
Fig. 6 Efficiency of SDT improved by constructing heterojunction or introducing defects on piezoelectric semiconductor nanomaterials[42-43] (a) i: Schematic diagram of the preparation and SDT treatment with Au@BP, ii: Time-dependent fluorescence of singlet oxygen sensor green (SOSG) under ultrasound irradiation, iii: Intracellular ROS level after different treatments[42] with (1-6) indicate blank, ultrasound, BP nanosheets, Au@BP nanohybrids, BP nanosheets with ultrasound, and Au@BP nanohybrids with ultrasound, respectively; (b) i: Schematic illustration of D-ZnOx:Gd under ultrasound irradiation, ii: Strucure of defect-free ZnO and defect-rich D-ZnOx:Gd and their adsorption energies with O2 and H2O [43] BP: Black phosphorus; CB: Conduction band; VB: Valence band
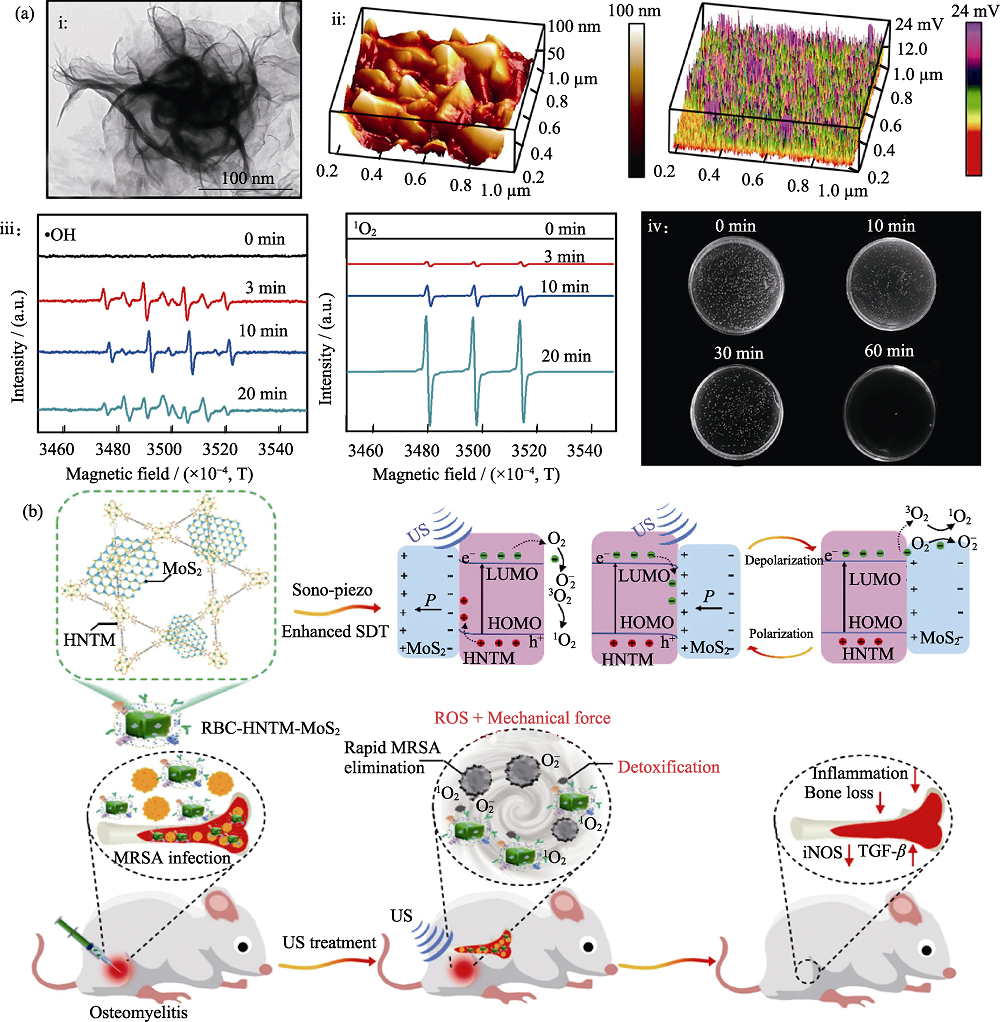
图7 压电半导体纳米材料在抗菌中的应用[44-45]
Fig. 7 Application of piezoelectric semiconductor nanomaterials in anti-bacterial[44-45] (a) i: HRTEM image of WS2 NFs, ii: Piezo force microscopy image and 3D piezoelectric potential image of WS2 NFs, iii: •OH and 1O2 were measured by electron spin-resonance spectroscopy (EPR), iv: Antibacterial properties of WS2 NFs against E. coli after ultrasound treatment; (b) Sonodynamic mechanism of porphyrin-based hollow metal-organic framework-MoS2 (HNTM-MoS2) and therapy on osteomyelitis; MRSA: Methicillin-resistant S. aureus; LUMO: Lowest unoccupied molecular orbital; HOMO: Highest occupied molecular orbital; HNTM: Hollow metal-organic framework; RBC: Red blood cell; iNOS: Inducible nitric oxide synthase; TGF-β: Transforming growth factor-β
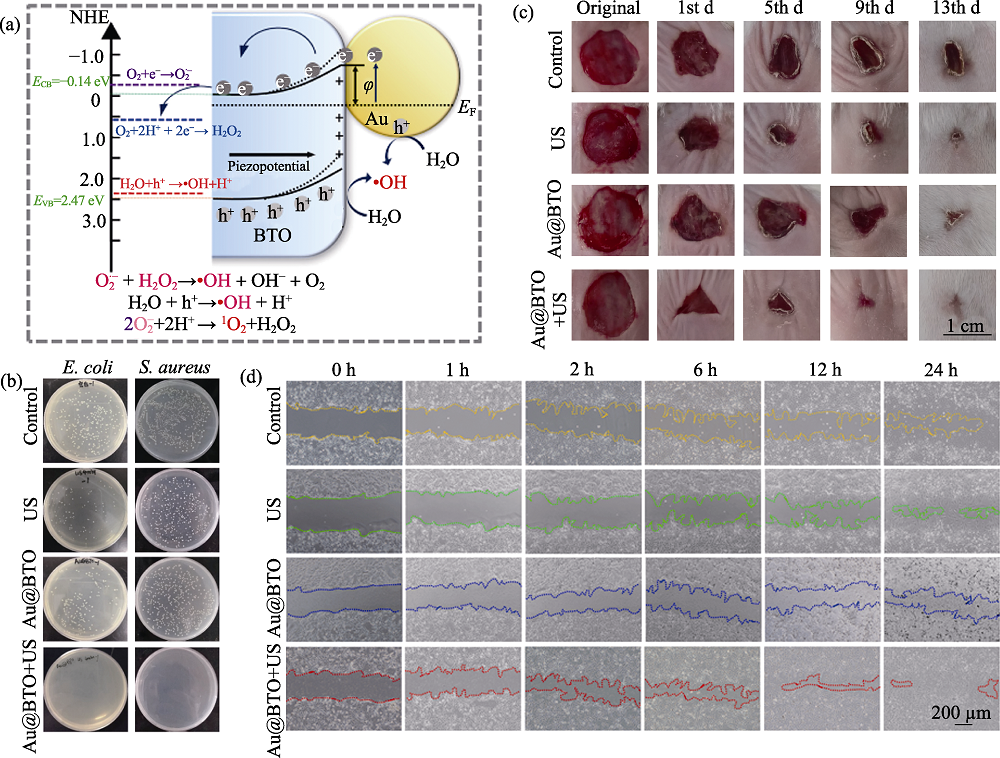
图8 Au@BTO用于抗菌和创口修复[46]
Fig. 8 Au@BTO for bacterial elimination and wound healing[46] (a) Mechanism of sonodynamic therapy using Au@BTO under ultrasound irradiation; (b) Sonodynamic antibacterial effect of Au@BTO against E. coli and S. aureus; (c) Representative photographs of mouse S. aureus infected wounds at different time (d) Representative images of NIH-3T3 cell migration; NHE: Normal hydrogen electrode; US: Ultrasound
| [1] |
BODEGA G, ALIQUE M, PUEBLA L, et al. Microvesicles: ROS scavengers and ROS producers. Journal of Extracellular Vesicles, 2019, 8(1): 1626654-10.
DOI URL |
| [2] | CAO Z, LI D, WANG J, et al. Reactive oxygen species-sensitive polymeric nanocarriers for synergistic cancer therapy. Acta Biomaterialia, 2021, 130: 17-31. |
| [3] | HE Y, HUA LIU S, YIN J, et al. Sonodynamic and chemodynamic therapy based on organic/organometallic sensitizers. Coordination Chemistry Reviews, 2021, 429: 213610-21. |
| [4] |
YANG B, CHEN Y, SHI J. Reactive oxygen species (ROS)-based nanomedicine. Chemical Reviews, 2019, 119(8): 4881-4985.
DOI PMID |
| [5] |
YUMITA N, NISHIGAKI R, UMEMURA K, et al. Hematoporphy- rin as a sensitizer of cell-damaging effect of ultrasound. Japanese Journal of Cancer Research, 1989, 80(3): 219-222.
DOI URL |
| [6] | LIU R, ZHANG Q, LANG Y, et al. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagnosis and Photodynamic Therapy, 2017, 19: 159-166. |
| [7] |
WANG H, PAN X, WANG X, et al. Degradable carbon-silica nano-composite with immunoadjuvant property for dual-modality photother-mal/photodynamic therapy. ACS Nano, 2020, 14(3): 2847-2859.
DOI URL |
| [8] |
YAO S, ZHAO X, WAN X, et al. π-π conjugation promoted nano-catalysis for cancer therapy based on a covalent organic framework. Materials Horizons, 2021, 8(12): 3457-3467.
DOI URL |
| [9] |
DEEPAGAN V G, YOU D G, UM W, et al. Long-circulating Au- TiO2 nanocomposite as a sonosensitizer for ROS-mediated eradication of cancer. Nano Letters, 2016, 16(10): 6257-6264.
DOI URL |
| [10] |
GONG F, CHENG L, YANG N, et al. Ultrasmall oxygen-deficient bimetallic oxide MnWOx nanoparticles for depletion of endogenous GSH and enhanced sonodynamic cancer therapy. Advanced Materials, 2019, 31(23): 1900730-9.
DOI URL |
| [11] |
ZHONG X, WANG X, CHENG L, et al. GSH-depleted PtCu3 nanocages for chemodynamic-enhanced sonodynamic cancer therapy. Advanced Functional Materials, 2020, 30(4): 1907954-12.
DOI URL |
| [12] |
ZHANG H, PAN X, WU Q, et al. Manganese carbonate nanoparti-cles-mediated mitochondrial dysfunction for enhanced sonodynamic therapy. Exploration, 2021, 1(2): 20210010-12.
DOI URL |
| [13] |
ZHU L, WANG Z L. Recent progress in piezo-phototronic effect enhanced solar cells. Advanced Functional Materials, 2019, 29(41): 1808214-18.
DOI URL |
| [14] |
CHORSI M T, CURRY E J, CHORSI H T, et al. Piezoelectric biomaterials for sensors and actuators. Advanced Materials, 2019, 31(1): 1802084-15.
DOI URL |
| [15] |
XU Q, GAO X, ZHAO S, et al. Construction of bio-piezoelectric platforms: from structures and synthesis to applications. Advanced Materials, 2021, 33(27): 2008452-28.
DOI URL |
| [16] |
WANG W, XU M, XU X, et al. Perovskite oxide based electrodes for high-performance photoelectrochemical water splitting. Angewandte Chemie International Edition, 2020, 59(1): 136-152.
DOI URL |
| [17] | YU X, WANG S, ZHANG X, et al. Heterostructured nanorod array with piezophototronic and plasmonic effect for photodynamic bacteria killing and wound healing. Nano Energy, 2018, 46: 29-38. |
| [18] |
MANNA S, TALLEY K R, GORAI P, et al. Enhanced piezoelectric response of AlN via CrN alloying. Physical Review Applied, 2018, 9(3): 34026-15.
DOI URL |
| [19] |
WANG Z L. Progress in piezotronics and piezo-phototronics. Advanced Materials, 2012, 24(34): 4632-4646.
DOI URL |
| [20] | PANDEY R K, DUTTA J, BRAHMA S, et al. Review on ZnO- based piezotronics and piezoelectric nanogenerators: aspects of pie-zopotential and screening effect. Journal of Physics: Materials, 2021, 4: 44011-22. |
| [21] |
GHASEMIAN M B, DAENEKE T, SHAHRBABAKI Z, et al. Peculiar piezoelectricity of atomically thin planar structures. Nanoscale, 2020, 12(5): 2875-2901.
DOI PMID |
| [22] |
HINCHET R, KHAN U, FALCONI C, et al. Piezoelectric properties in two-dimensional materials: simulations and experiments. Materials Today, 2018, 21(6): 611-630.
DOI URL |
| [23] | WU J M. Piezo-catalytic effect on the enhancement of the ultra- high degradation activity in the dark by single- and few-layers MoS2 nanoflowers. Advanced Matericals, 2016, 28(19): 3718-3725. |
| [24] | WANG Z L, WILLATZEN M. Prediction of strong piezoelectricity in 3R-MoS2 multilayer structures. Nano Energy, 2019, 56: 512-515. |
| [25] |
UM W, E K P K, LEE J, et al. Recent advances in nanomaterial- based augmented sonodynamic therapy of cancer. Chemical Communications, 2021, 57(23): 2854-2866.
DOI URL |
| [26] |
XU H, SUSLICK K S. Molecular emission and temperature meas- urements from single-bubble sonoluminescence. Physical Review Letters, 2010, 104(24): 244301-4.
DOI URL |
| [27] |
DIDENKO Y T, SUSLICK K S. The energy efficiency of formation of photons, radicals and ions during single-bubble cavitation. Nature, 2002, 418(6896): 394-397.
DOI URL |
| [28] |
NOSAKA Y, NOSAKA A Y. Generation and detection of reactive oxygen species in photocatalysis. Chemical Reviews, 2017, 117(17): 11302-11336.
DOI PMID |
| [29] |
LI Y, XIE J, UM W, et al. Sono/photodynamic nanomedicine-elicited cancer immunotherapy. Advanced Functional Materials, 2021, 31(12): 2008061-25.
DOI URL |
| [30] | CURIE J, CURIE P. Développement par compression de l’électricité polaire dans les cristaux hémièdres à faces inclinées. Bulletin de la Société Chimique de France, 1880, 91: 294-295. |
| [31] |
WU J, MAO W, WU Z, et al. Strong pyro-catalysis of pyroelectric BiFeO3 nanoparticles under a room-temperature cold-hot alternation. Nanoscale, 2016, 8(13): 7343-7350.
DOI URL |
| [32] |
WANG Y, WEN X, JIA Y, et al. Piezo-catalysis for nondestructive tooth whitening. Nature Communications, 2020, 11(1): 1328-11.
DOI PMID |
| [33] |
WANG Z L. Piezopotential gated nanowire devices: piezotronics and piezo-phototronics. Nano Today, 2010, 5(6): 540-552.
DOI URL |
| [34] |
PAN L, SUN S, CHEN Y, et al. Advances in piezo-phototronic effect enhanced photocatalysis and photoelectrocatalysis. Advanced Energy Materials, 2020, 10(15): 2000214-25.
DOI URL |
| [35] |
KANG Y, LEI L, ZHU C, et al. Piezo-photocatalytic effect mediat- ing reactive oxygen species burst for cancer catalytic therapy. Materials Horizons, 2021, 8(8): 2273-2285.
DOI URL |
| [36] |
ZHU L, WANG Z L. Progress in piezotronics and piezo-phototronics of quantum materials. Journal of Physics D: Applied Physics, 2019, 52(34): 343001-25.
DOI URL |
| [37] | ZHOU Z, YUAN S, WANG J. Theoretical progress on direct z-scheme photocatalysis of two-dimensional heterostructures. Frontiers of Physics, 2021, 16(4): 1-9. |
| [38] |
ZHOU P, YU J, JARONIEC M. All-solid-state z-scheme photocata- lytic systems. Advanced Materials, 2014, 26(29): 4920-4935.
DOI URL |
| [39] |
LI Z, ZHANG T, FAN F, et al. Piezoelectric materials as sono- dynamic sensitizers to safely ablate tumors: a case study using black phosphorus. Journal of Physical Chemistry Letters, 2020, 11(4): 1228-1238.
DOI URL |
| [40] |
ZHU P, CHEN Y, SHI J. Piezocatalytic tumor therapy by ultrasound-triggered and BaTiO3-mediated piezoelectricity. Advanced Materials, 2020, 32(29): 2001976-8.
DOI URL |
| [41] |
DONG Y, DONG S, LIU B, et al. 2D piezoelectric Bi2MoO6nano- ribbons for GSH-enhanced sonodynamic therapy. Advanced Materials, 2021, 33(51): 2106838-11.
DOI URL |
| [42] |
OUYANG J, DENG L, CHEN W, et al. Two dimensional semicon- ductors for ultrasound-mediated cancer therapy: the case of black phos-phorus nanosheets. Chemical Communications, 2018, 54(23): 2874-2877.
DOI URL |
| [43] | LIU Y, WANG Y, ZHEN W, et al. Defect modified zinc oxide with augmenting sonodynamic reactive oxygen species generation. Biomaterials, 2020, 251: 120075-9. |
| [44] | MASIMUKKU S, HU Y C, LIN Z H, et al. High efficient degradation of dye molecules by PDMS embedded abundant singlelayer tungsten disulfide and their antibacterial performance. Nano Energy, 2018, 46: 338-346. |
| [45] |
FENG X, MA L, LEI J, et al. Piezo-augmented sonosensitizer with strong ultrasound-propelling ability for efficient treatment of osteomye-litis. ACS Nano, 2022, 16(2): 2546-2557.
DOI URL |
| [46] | WU M, ZHANG Z, LIU Z, et al. Piezoelectric nanocomposites for sonodynamic bacterial elimination and wound healing. Nano Today, 2021, 37: 101104-12. |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [8] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [9] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [10] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [11] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [12] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| [13] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| [14] | 陶桂龙, 支国伟, 罗添友, 欧阳佩东, 衣新燕, 李国强. 空腔型薄膜体声波滤波器的关键技术进展[J]. 无机材料学报, 2025, 40(2): 128-144. |
| [15] | 周帆, 田志林, 李斌. 热防护系统用碳化物超高温陶瓷抗烧蚀涂层研究进展[J]. 无机材料学报, 2025, 40(1): 1-16. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||