无机材料学报 ›› 2022, Vol. 37 ›› Issue (4): 395-403.DOI: 10.15541/jim20210255 CSTR: 32189.14.10.15541/jim20210255
马磊1,2( ), 黄毅1(
), 黄毅1( ), 邓浩2, 银航1,2, 田强1, 晏敏皓1
), 邓浩2, 银航1,2, 田强1, 晏敏皓1
收稿日期:2021-04-15
修回日期:2021-05-27
出版日期:2022-04-20
网络出版日期:2021-06-30
通讯作者:
黄毅, 讲师. E-mail: huangyi516@163.com作者简介:马磊(1996-), 男, 硕士研究生. E-mail: 2219835537@qq.com
基金资助:
MA Lei1,2( ), HUANG Yi1(
), HUANG Yi1( ), DENG Hao2, YIN Hang1,2, TIAN Qiang1, YAN Minghao1
), DENG Hao2, YIN Hang1,2, TIAN Qiang1, YAN Minghao1
Received:2021-04-15
Revised:2021-05-27
Published:2022-04-20
Online:2021-06-30
Contact:
HUANG Yi, lecturer. E-mail: huangyi516@163.comAbout author:MA Lei(1996-), male, Master candidate. E-mail: 2219835537@qq.com
Supported by:摘要:
随着全球核能的开发利用, 铀已成为土壤、地表水和地下水的常见污染物, 从含铀废水中去除铀(VI)已成为迫切需要。本工作以氟化钙、焦磷酸钙、氢氧化钙为反应原料合成氟磷灰石, 系统研究了其对铀(VI)的去除性能并采用不同测试手段对吸附铀(VI)前后的氟磷灰石进行表征, 揭示了其相关去除机理。结果表明: 在温度为308 K, pH=3, 固液比为0.12 g/L, 平衡时间为120 min, 初始铀浓度为100 mg/L的条件下, 氟磷灰石对铀(VI)的吸附容量可达655.17 mg/g, 其吸附过程符合准二级动力学和Langmuir等温吸附模型, 且为自发和吸热过程。氟磷灰石对铀(VI)的去除机理为表面矿化, 吸附铀(VI)的氟磷灰石表面产生了新相准钙铀云母[Ca(UO2)2(PO4)2·6H2O], 准钙铀云母在pH≥3水溶液中能保持较高稳定性。因此, 氟磷灰石可以作为一种有前景的矿化剂, 用于含铀废水的净化和固体化处理。
中图分类号:
马磊, 黄毅, 邓浩, 银航, 田强, 晏敏皓. 氟磷灰石对酸性水溶液中铀(VI)的去除研究[J]. 无机材料学报, 2022, 37(4): 395-403.
MA Lei, HUANG Yi, DENG Hao, YIN Hang, TIAN Qiang, YAN Minghao. Removal of Uranium (VI) from Acidic Aqueous Solution by Fluorapatite[J]. Journal of Inorganic Materials, 2022, 37(4): 395-403.

图1 合成氟磷灰石粉体XRD图谱(a), FT-IR谱图 (b), 粒径分布图(c)和SEM照片(d)
Fig. 1 XRD pattern(a), FT-IR spectrum(b), particle size distribution (c) and SEM image (d) of synthetic fluorapatite powder

图2 (a)铀(VI)在不同pH水溶液中的种态分布图(C0=100 mg/L, T=308 K); (b)不同pH水溶液中氟磷灰石对铀(VI)的吸附容量和去除率; (c)不同pH水溶液中氟磷灰石的Zeta电位; (d)固液比对氟磷灰石吸附铀(VI)的影响(pH=3); (e)氟磷灰石吸附铀(VI)随吸附时间的变化(pH=3, 固液比0.12 g/L); (f)初始铀(VI)浓度对氟磷灰石吸附铀(VI)的影响(pH=3, 固液比0.12 g/L, 吸附时间120 min)
Fig. 2 (a) Distribution of uranium (VI) species in the solution with different pH (C0=100 mg/L, T= 308 K); (b) Adsorption capacity and removal rate of uranium (VI) by fluorapatite in the solution with different pH; (c) Zeta potential of fluorapatite in the solution with different pH; (d) Effect of solid-liquid ratio on adsorption of uranium (VI) by fluorapatite (pH3); (e) Change of the adsorption of uranium (VI) by fluorapatite with adsorption time (pH3, solid-liquid ratio at 0.12 g/L); (f) Effect of initial uranium (VI) concentration on adsorption of uranium (VI) by fluorapatite (pH3, solid-liquid ratio at 0.12 g/L, adsorption time=120 min)

图3 氟磷灰石(a)和吸附铀(VI)后的氟磷灰石(b)在不同pH水溶液中的溶解性
Fig. 3 Solubility of fluorapatite (a) and fluorapatite adsorbed with uranium (VI) (b) in the solutions with different pH
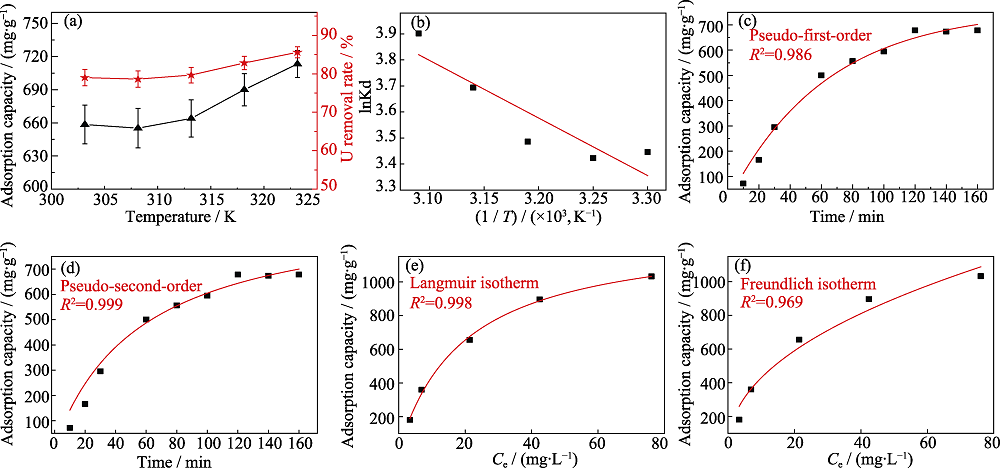
图4 (a)303~323 K吸附温度范围, 氟磷灰石对铀(VI)的吸附容量和去除率; (b)吸附过程的热力学模型的线性拟合; (c)准一级动力学模型的非线性拟合; (d)准二级动力学模型的非线性拟合; (e)Langmuir等温吸附模型的非线性拟合; (f) Freundlich等温吸附模型的非线性拟合
Fig. 4 (a) Adsorption capacity and removal rate of uranium (VI) by fluorapatite in the temperature range of 303-323 K; (b) Linear fitting of thermodynamic model for adsorption process; (c) Nonlinear fitting of pesudo-first-order kinetic model; (d) Nonlinear fitting of pesudo-second-order kinetic model; (e) Nonlinear fitting of Langmuir isotherm adsorption model; (f) Nonlinear fitting of Freundlich isotherm adsorption model
| Materials | ∆H/(kJ∙mol-1) | ∆S/(J∙mol-1•K-1) | ∆G/(kJ•mol-1) | ||||
|---|---|---|---|---|---|---|---|
| 303 K | 308 K | 313 K | 318 K | 323 K | |||
| Fluorapatite | 8.41 | 88.65 | -8.47 | -8.91 | -9.31 | -9.8 | -10.24 |
表1 吸附过程热力学模型的拟合参数
Table 1 Fitting parameters of thermodynamic model for adsorption
| Materials | ∆H/(kJ∙mol-1) | ∆S/(J∙mol-1•K-1) | ∆G/(kJ•mol-1) | ||||
|---|---|---|---|---|---|---|---|
| 303 K | 308 K | 313 K | 318 K | 323 K | |||
| Fluorapatite | 8.41 | 88.65 | -8.47 | -8.91 | -9.31 | -9.8 | -10.24 |
| Materials | Pseudo-first-order | Pseudo-second-order | ||
|---|---|---|---|---|
| K1/min-1 | R2 | K2 / (g∙mg-1∙min-1) | R2 | |
| Fluorapatite | 0.016 | 0.986 | 1.85×10-5 | 0.999 |
表2 准一级和准二级动力学模型的拟合参数
Table 2 Fitting parameters of pesudo-first-order and pesudo-second-order kinetic models
| Materials | Pseudo-first-order | Pseudo-second-order | ||
|---|---|---|---|---|
| K1/min-1 | R2 | K2 / (g∙mg-1∙min-1) | R2 | |
| Fluorapatite | 0.016 | 0.986 | 1.85×10-5 | 0.999 |
| Materials | Langmuir | Freundlich | |||
|---|---|---|---|---|---|
| qmax /(mg∙g-1) | R2 | n | R2 | ||
| Fluorapatite | 1300.35 | 0.998 | 2.18 | 0.969 | |
表3 Langmuir和Freundlich等温吸附模型的拟合参数
Table 3 Fitting parameters of Langmuir and Freundlich isothermal adsorption models
| Materials | Langmuir | Freundlich | |||
|---|---|---|---|---|---|
| qmax /(mg∙g-1) | R2 | n | R2 | ||
| Fluorapatite | 1300.35 | 0.998 | 2.18 | 0.969 | |
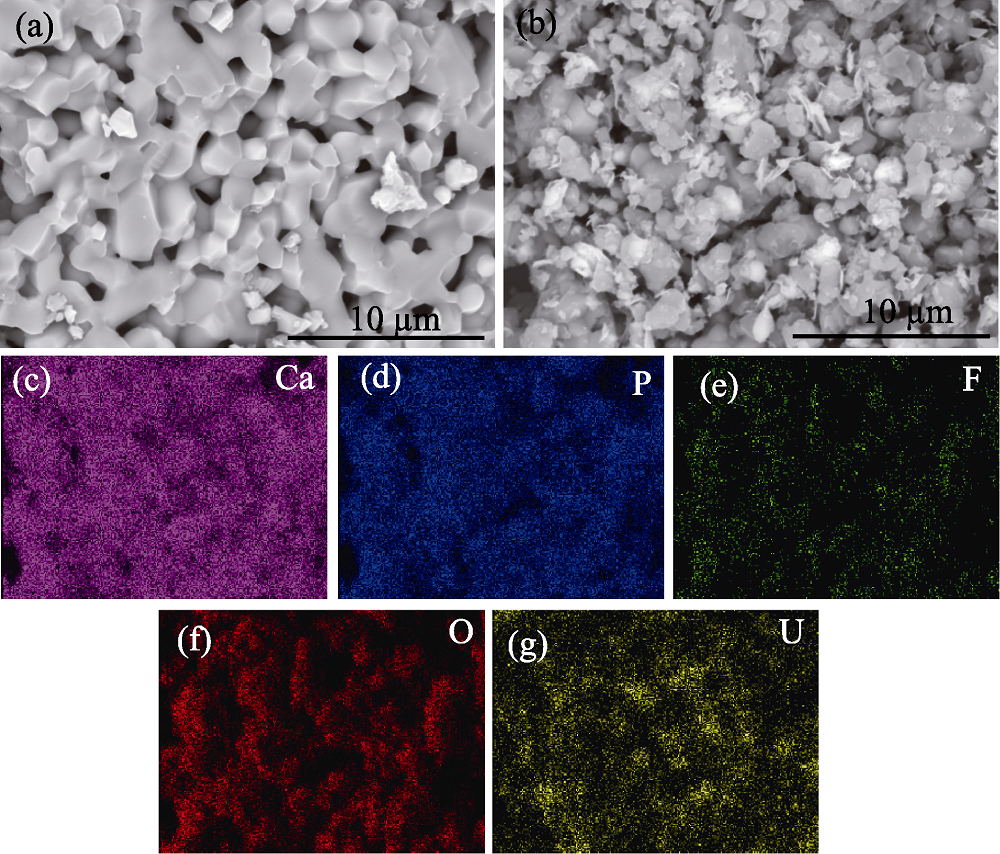
图5 吸附铀(VI)前(a)后(b)的氟磷灰石SEM照片和吸附铀(VI)的氟磷灰石表面元素分布图(c~g)
Fig. 5 SEM images of fluorapatite before (a) and after (b) adsorption of uranium (VI), and element mappings (c-g) of the surface of fluorapatite after adsorption of uranium (VI)
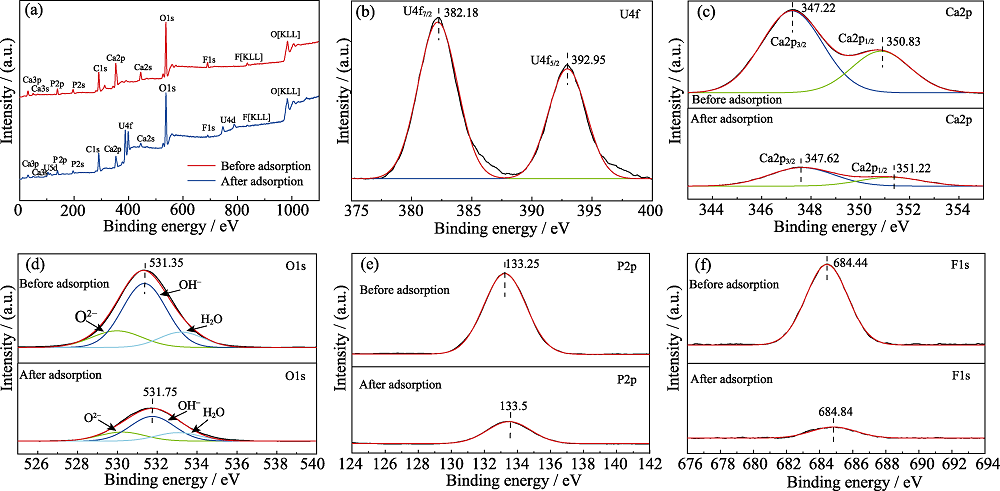
图6 氟磷灰石吸附铀(VI)前后(a)和氟磷灰石吸附铀(VI)后U4f(b)的XPS谱图; 氟磷灰石吸附铀(VI))前后Ca2p(c)、O1s(d)、 P2p(e)和F1s(f)的XPS谱图
Fig. 6 (a) XPS spectra of fluorapatite before and after adsorption of uranium (VI) and (b) U4f on the fluorapatite after adsorption of uranium (VI) (b), and XPS spectra of Ca2p(c), O1s(d), P2p(e) and F1s(f) on the fluorapatite before and after adsorption of uranium (VI)
| [1] |
GIANNAKOUDAKIS D A, ANASTOPOULOS I, BARCZAK M, et al. Enhanced uranium removal from acidic wastewater by phosphonate-functionalized ordered mesoporous silica: surface chemistry matters the most. Journal of Hazardous Materials, 2021, 413: 125279.
DOI URL |
| [2] | WANG J Q, PANG H W, TANG H, et al. Study on the removal of U(VI) in water by carbon supported zero valent iron prepared by carbothermal reduction method. Journal of Inorganic Materials, 2020, 35(3): 373-380. |
| [3] | ZHANG Z B, ZHOU R Z, DONG Z M, et al. Effect of amidoxime hydrothermal carbon on U(VI)-CO3/Ca- U(VI)-CO3. Journal of Inorganic Materials, 2020, 35(03): 352-358. |
| [4] |
SALAMA E, EL-KAMEESY S U, ELRAWI R. Depleted uranium assessment and natural radioactivity monitoring in north west of Iraq over a decade since the last gulf war. Journal of Environmental Radioactivity, 2019, 201: 25-31.
DOI URL |
| [5] |
VENU-BABU P, SUSAN E. High efficiency phytoextraction of uranium using vetiveria zizanioides L. Nash. International Journal of Phytoremediation, 2020, 22(11): 1137-1146.
DOI URL |
| [6] |
YANG S Y, LI Q, CHEN L, et al. Synergistic removal and reduction of U(VI) and Cr(VI) by Fe3S4 micro-crystal. Chemical Engineering Journal, 2020, 385: 123909.
DOI URL |
| [7] |
WANG X L, THOMAS M J, CRAIG C L. Low temperature equilibrium isotope fractionation and isotope exchange kinetics between U(IV) and U(VI). Geochimica et Cosmochimica Acta, 2015, 158: 262-275.
DOI URL |
| [8] |
SUDESHNA S, HIRAKENDU B, ROUT S. et al. Nanohydroxyapatite coated activated carbon impregnated alginate: a new hybrid sorbent for uranium removal from potable water. Journal of Environmental Chemical Engineering, 2020, 8(4):103999.
DOI URL |
| [9] |
YU S J, WANG X X, LIU Y F, et al. Efficient removal of uranium(VI) by layered double hydroxides supported nanoscale zero-valent iron: a combined experimental and spectroscopic studies. Chemical Engineering Journal, 2019, 365: 51-59.
DOI URL |
| [10] |
DAI Y M, ZHOU L M, TANG X H, et al. Macroporous ion-imprinted chitosan foams for the selective biosorption of U(VI) from aqueous solution. International Journal of Biological Macromolecules, 2020, 164: 4155-4164.
DOI URL |
| [11] |
MA D H, WEI J J, ZHAO Y S, et al. The removal of uranium using novel temperature sensitive urea-formaldehyde resin: adsorption and fast regeneration. Science of the Total Environment, 2020, 735: 139399.
DOI URL |
| [12] |
LU W, DAI Z R, LI L, et al. Preparation of composite hydrogel (PCG)and its adsorption performance for uranium(VI). Journal of Molecular Liquids, 2020, 303: 112604.
DOI URL |
| [13] |
WU Y H, CHEN D Y, KONG L J, et al. Rapid and effective removal of uranium(VI) from aqueous solution by facile synthesized hierarchical hollow hydroxyapatite microspheres. Journal of Hazardous Materials, 2019, 371: 397-405.
DOI URL |
| [14] |
SHI Q P, SU M H, YUVARAJA G, et al. Development of highly efficient bundle-like hydroxyapatite towards abatement of aqueous U(VI) ions: mechanism and economic assessment. Journal of Hazardous Materials, 2020, 394: 122550.
DOI URL |
| [15] |
HAN R P, ZOU W H, WANG Y, et al. Removal of uranium (VI) from aqueous solutions by manganese oxide coated zeolite: discussion of adsorption isotherms and pH effect. Journal of Environmental Radioactivity, 2007, 93(3): 127-143.
DOI URL |
| [16] |
CAMACHO L M, DENG S G, PARRA R R. Uranium removal from groundwater by natural clinoptilolite zeolite: effects of pH and initial feed concentration. Journal of Hazardous Materials, 2010, 175(1/2/3): 393-398.
DOI URL |
| [17] |
ZONG P F, WU X Y, GOU J Y, et al. Immobilization and recovery of uranium (VI) using Na-bentonite from aqueous medium: equilibrium, kinetics and thermodynamics studies. Journal of Molecular Liquids, 2015, 209: 358-366.
DOI URL |
| [18] |
HOUHOUNE F, NIBOU D, CHEGROUCHE S, et al. Behaviour of modified hexadecyltrimethylammonium bromide bentonite toward uranium species. Journal of Environmental Chemical Engineering, 2016, 4(3): 3459-3467.
DOI URL |
| [19] |
MARIA E, IOANNIS P. A comparative study of the adsorption of uranium on commercial and natural (Cypriot) sea sand samples. Journal of Radioanalytical and Nuclear Chemistry, 2013, 298(2): 1111-1116.
DOI URL |
| [20] |
HU W, ZHANG Z X, LI M X, et al. Enhanced uptake capacity for uranium (VI) in aqueous solutions by activated natural siderite: performance and mechanism. Applied Geochemistry, 2019, 100: 96-103.
DOI URL |
| [21] |
BENGTSSON A, SHCHUKAREV A, PERSSON P, et al. Phase transformations, ion-exchange, adsorption, and dissolution processes in aquatic fluorapatite systems. Langmuir, 2009, 25(4): 2355-2362.
DOI URL |
| [22] | VALYASHKO V M, KOGARKO L N, KHODAKOVSKIY I L. Stability of fluorapatite, chlorapatite and hydroxylapatite in aqueous solutions at different temperatures. Geochemistry International, 1968, 5(1): 21-30. |
| [23] |
HUANG Y, ZHANG H B, ZHOU X S, et al. Synthesis and microstructure of fluorapatite-type Ca10-2xSmxNax(PO4)6F2 solid solutions for immobilization of trivalent minor actinide. Journal of Nuclear Materials, 2017, 485: 105-112.
DOI URL |
| [24] |
BROS R, CARPENA J, SERE V, et al. Occurrence of Pu and fissiogenic REE in hydrothermal apatites from the fossil nuclear reactor 16 at Oklo (Gabon). Radiochimica Acta, 2013, 74(s1): 277-282.
DOI URL |
| [25] |
CHAUMONT J, SOULET S, KRUPA J C, et al. Competition between disorder creation and annealing in fluoroapatite nuclear waste forms. Journal of Nuclear Materials, 2002, 301(2): 122-128.
DOI URL |
| [26] |
MORENO E C, KRESAK M, ZAHRADNIK R T. Fluoridated hydroxyapatite solubility and caries formation. Nature, 1974, 247(5435): 64-65.
DOI URL |
| [27] |
GAO X N, HUANG Y, TENG Y C, et al. Fabrication and chemical durability of hot-pressed Na-bearing fluorapatite-type Ca8Sm1Na1(PO4)6F2 ceramic for immobilization of trivalent minor actinide. Journal of Nuclear Materials, 2018, 507: 297-305.
DOI URL |
| [28] |
OHNUKI T, KOZAI N, SAMADFAM M, et al. The formation of autunite (Ca(UO2)2(PO4)2•nH2O) within the leached layer of dissolving apatite: incorporation mechanism of uranium by apatite. Chemical Geology, 2004, 211(1): 1-14.
DOI URL |
| [29] |
FATHI M H, MOHAMMADI Z E. Mechanical alloying synthesis and bioactivity evaluation of nanocrystalline fluoridated hydroxyapatite. Journal of Crystal Growth, 2008, 311(5): 1392-1403.
DOI URL |
| [30] |
TROMMER R M, SANTOS L A, BERGMANN C P. Alternative technique for hydroxyapatite coatings. Surface & Coatings Technology, 2007, 201(24): 9587-9593.
DOI URL |
| [31] |
HAN M N, KONG L J, HU X L, et al. Phase migration and transformation of uranium in mineralized immobilization by wasted bio-hydroxyapatite. Journal of Cleaner Production, 2018, 197: 886-894.
DOI URL |
| [32] |
WOJCIECH P, WLADYSLAW R, ANITA P. Theoretical models of sorption kinetics including a surface reaction mechanism: a review. Advances in Colloid and Interface Science, 2009, 152(1): 2-13.
DOI URL |
| [33] |
LANGMUIR I. The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 1918, 40(9): 1361-1403.
DOI URL |
| [34] | FREUNDLICH H. Über die adsorption in lösungen. Zeitschrift für Physikalische Chemie, 1906, 57: 385-471. |
| [35] |
ZHANG Z X, LIU H B, SONG W C, et al. Accumulation of U(VI) on the Pantoea sp. TW18 isolated from radionuclide-contaminated soils. Journal of Environmental Radioactivity, 2018, 192: 219-226.
DOI URL |
| [36] |
LI M X, LIU H B, CHEN T H, et al. Synthesis of magnetic biochar composites for enhanced uranium (VI) adsorption. Science of the Total Environment, 2018, 651(P1): 1020-1028.
DOI URL |
| [37] |
ZHENG N C, YIN L Y, SU M H, et al. Synthesis of shape and structure-dependent hydroxyapatite nanostructures as a superior adsorbent for removal of U(VI). Chemical Engineering Journal, 2020, 384: 123262
DOI URL |
| [1] | 王婷婷, 史书梅, 柳晨媛, 朱万诚, 张恒. 多级多孔硅酸镍微球的合成及其对碱性品红的高效吸附[J]. 无机材料学报, 2021, 36(12): 1330-1336. |
| [2] | 宋环, 王琳, 王宏青, 石伟群. 碱化Ti3C2Tx MXene对Eu(III)高效去除与机理研究[J]. 无机材料学报, 2020, 35(1): 65-72. |
| [3] | 耿瑞文, 杨晓京, 谢启明, 李芮, 罗良. 基于划刻实验的单晶锗材料去除机理研究[J]. 无机材料学报, 2019, 34(8): 867-872. |
| [4] | 徐从斌, 杨文杰, 孙宏亮, 刘伟江, 杨苑钰, 林爱军. 膨胀石墨负载零价铁的合成及其对水中Pb(II)去除效果与机制[J]. 无机材料学报, 2018, 33(1): 41-47. |
| [5] | 刘守新,刘正锋. TiO2/ACF复合材料的Sol-Gel法制备及其对苯的去除性能[J]. 无机材料学报, 2009, 24(2): 209-214. |
| [6] | 叶彬,崔凯,冯庆玲,陈国强,崔福斋. 载银氟磷灰石抗菌剂的制备和耐高温性能研究[J]. 无机材料学报, 2003, 18(2): 485-489. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||