无机材料学报 ›› 2021, Vol. 36 ›› Issue (11): 1223-1230.DOI: 10.15541/jim20210142 CSTR: 32189.14.10.15541/jim20210142
收稿日期:2021-03-10
修回日期:2021-04-10
出版日期:2021-11-20
网络出版日期:2021-04-30
通讯作者:
王应德, 教授. E-mail: wangyingde@nudt.edu.cn;王 兵, 副研究员. E-mail: bingwang@nudt.edu.cn
作者简介:李鹏鹏(1995-), 男, 硕士研究生. E-mail: lipengpeng@nudt.edu.cn
LI Pengpeng( ), WANG Bing(
), WANG Bing( ), WANG Yingde(
), WANG Yingde( )
)
Received:2021-03-10
Revised:2021-04-10
Published:2021-11-20
Online:2021-04-30
Contact:
WANG Yingde, professor. E-mail: wangyingde@nudt.edu.cn;WANG Bing, associate professor. E-mail: bingwang@nudt.edu.cn
About author:LI Pengpeng(1995-), male, Master candidate. E-mail: lipengpeng@nudt.edu.cn
Supported by:摘要:
CO作为一种高毒性的气体,既是污染空气的元凶之一,长时间吸入也会对人体造成极大的伤害,甚至致死。如何实现CO的快速监测是传感领域面临的重要挑战。CO监测对保护人类健康和环境来说是一项必要的工作。在该研究中, 多孔CeO2纳米片(CeO2 NSs)通过火焰退火用简单水热法合成的中间产物CeOHCO3纳米片而得到。通过控制火焰退火时间, 可将氧空位引入到CeO2纳米片中。结果表明, 退火2 min得到的CeO2纳米片(CeO2-2min NSs)对CO气体表现出优异的重复性和选择性。尤其是, CeO2-2min NSs在450 ℃对500 μL/L CO的相应/恢复时间极快(2 s/2 s), 在宽范围内(10~10000 μL/L) CO浓度与响应值之间存在良好的函数关系。CeO2-2min NSs优秀的气敏性能可归因于多孔二维结构高的比表面积和晶体内丰富的氧空位。这项工作对设计检测宽范围气体的快响应气体传感器提供了借鉴。
中图分类号:
李鹏鹏, 王兵, 王应德. 基于火焰退火多孔CeO2纳米片的环境监测用超快CO气体传感器[J]. 无机材料学报, 2021, 36(11): 1223-1230.
LI Pengpeng, WANG Bing, WANG Yingde. Ultrafast CO Sensor Based on Flame-annealed Porous CeO2 Nanosheets for Environmental Application[J]. Journal of Inorganic Materials, 2021, 36(11): 1223-1230.
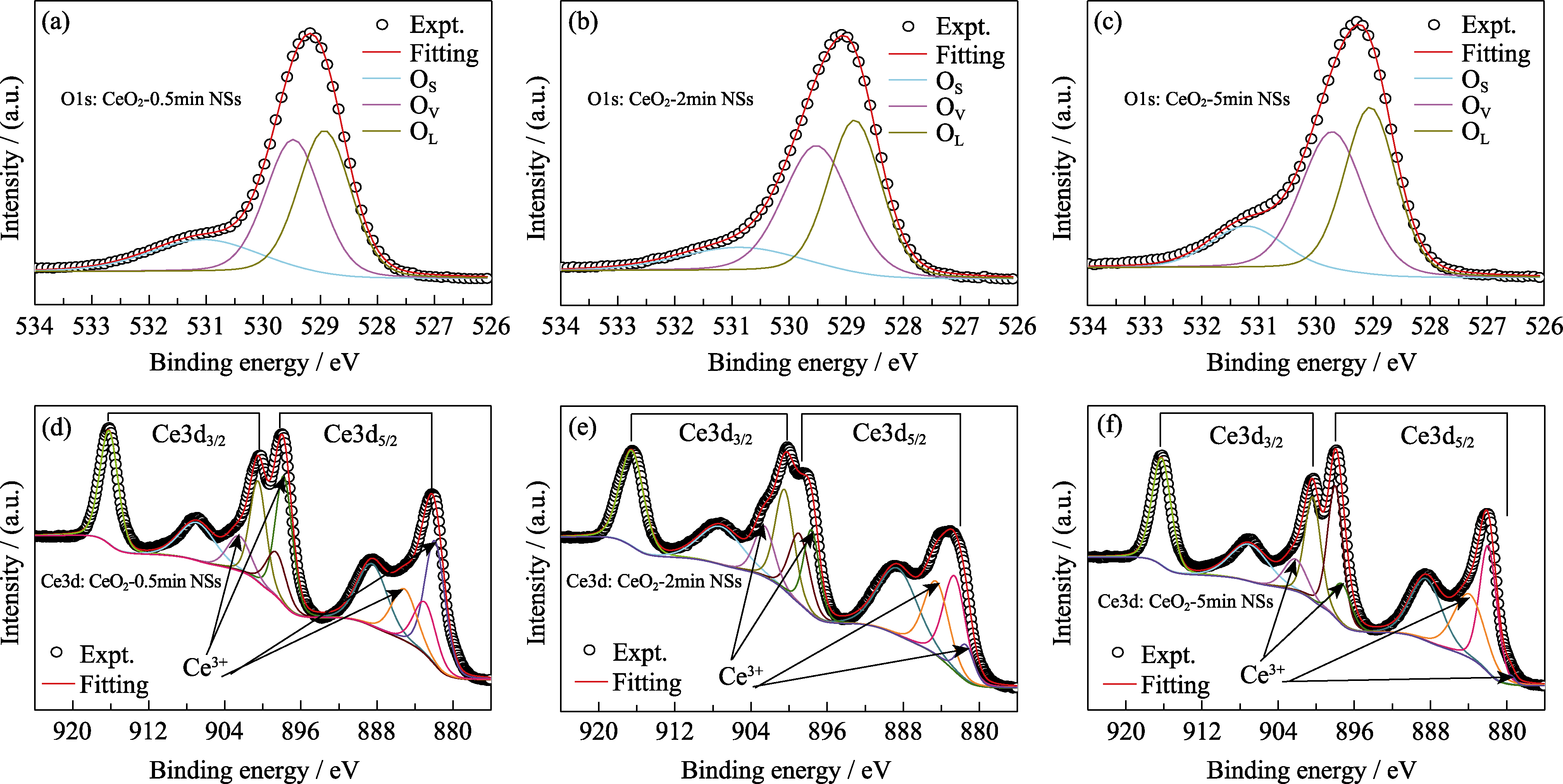
Fig. 3 XPS spectra of the CeO2 NSs (a, b, c) high resolution spectra of O 1s of CeO2-0.5min NSs, CeO2-2min NSs and CeO2-5min NSs; (d, e, f) high resolution spectra of Ce 3d of CeO2-0.5min NSs, CeO2-2min NSs and CeO2-5min NSs
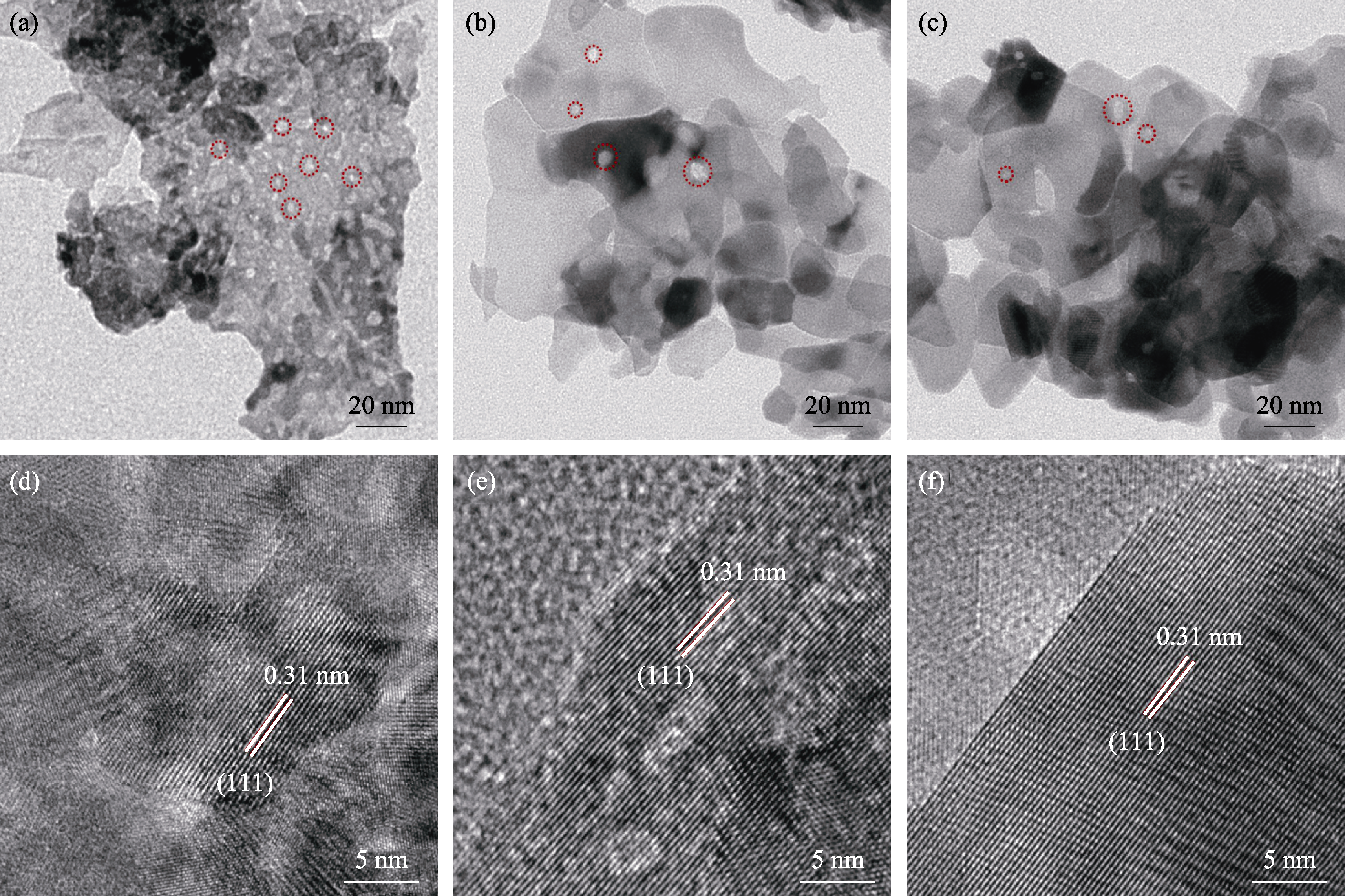
Fig. 4 Morphologies and structure characterizations of the as-prepared samples (a, b, c) TEM images of CeO2-0.5min NSs, CeO2-2min NSs and CeO2-5min NSs (pores are labelled by red dotted circles); (d, e, f) HRTEM images of CeO2-0.5min NSs, CeO2-2min NSs and CeO2-5min NSs
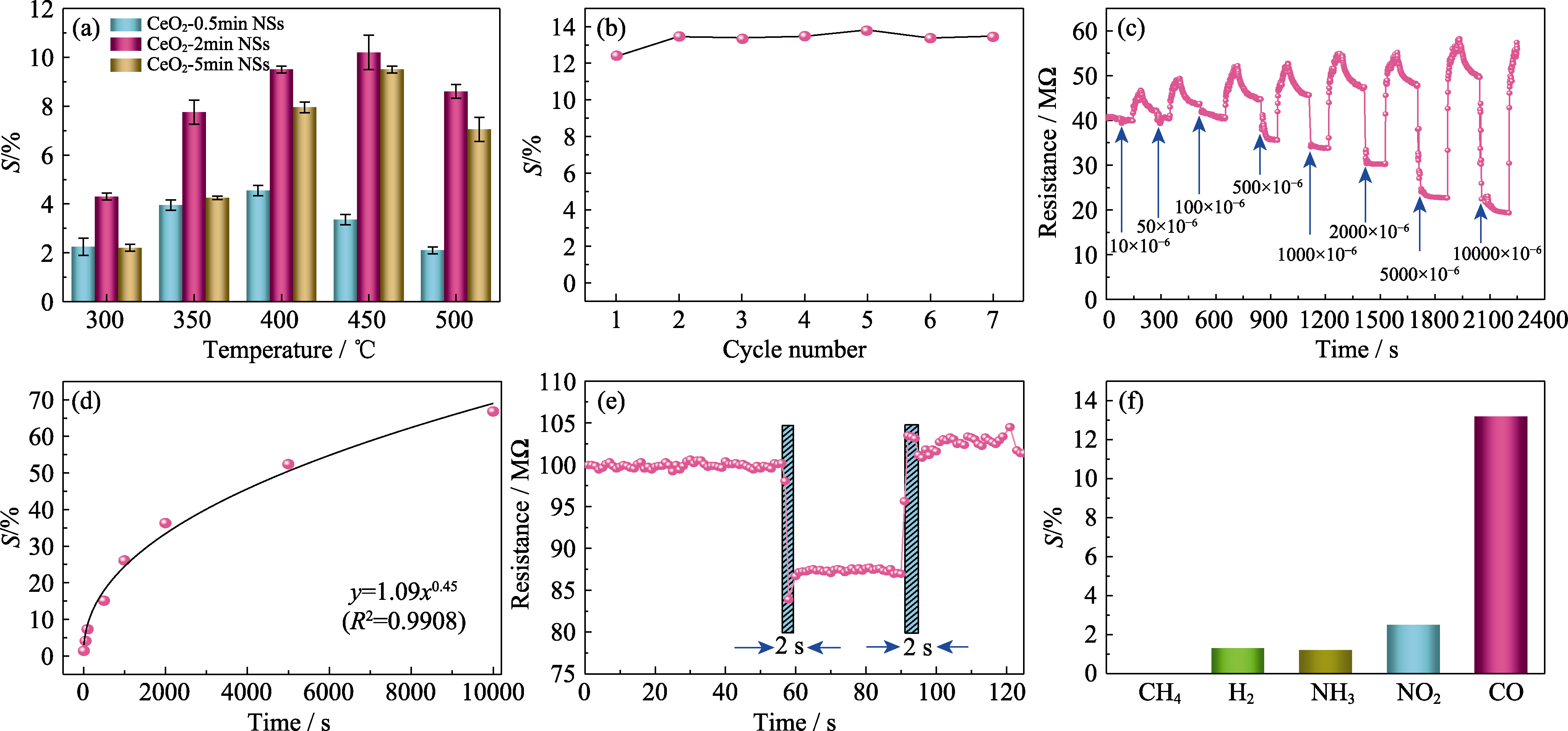
Fig. 7 Gas sensing properties of CeO2 NSs (a) Response of CeO2 NSs at different temperatures from 300 ℃ to 500 ℃ with an interval of 50 ℃ towards 500 μL/L CO; (b) Response of CeO2-2min NSs towards 500 μL/L CO at 450 ℃ for seven periods; (c) Transient response of CeO2-2min NSs towards different concentration from 10 μL/L to 10000 μL/L at 450 ℃; (d) Fitting of CO concentration and its corresponding response; (e) Determination of response/recovery time of CeO2-2min NSs towards 500 μL/L CO at 450 ℃; (f) Selectivity of CeO2-2min NSs towards CH4, H2, NH3, NO2 and CO at 450 ℃
| Material | Concentration/(μL∙L-1) | Temperature/℃ | Sensitivity | (Response/recovery time)/s | Ref. |
|---|---|---|---|---|---|
| Co3O4 nanostructures | 5 | 100 | 2.4a | 14/36 | [23] |
| Pd/SnO2 | 100 | 100 | 3.5a | 60/150 | [24] |
| TiO2-CeO2 mixed oxides | 400 | 200 | 10.7a | 32/45 | [25] |
| SnO2/MoO2 | 100 | RT | 9.2%b | 20/16 | [1] |
| SnO2-CeO2 mixed oxides | 500 | 430 | 190%b | 26/30 | [11] |
| Pd/SnO2 nanowires | 200 | 400 | 6.8a | 5/40 | [26] |
| ZnO nanorods | 30 | 400 | 1.1a | 46/27 | [27] |
| Porous CeO2 nanosheets | 500 | 450 | 12.0%b | 2/2 | This work |
Table 1 Recent materials for in CO gas sensing
| Material | Concentration/(μL∙L-1) | Temperature/℃ | Sensitivity | (Response/recovery time)/s | Ref. |
|---|---|---|---|---|---|
| Co3O4 nanostructures | 5 | 100 | 2.4a | 14/36 | [23] |
| Pd/SnO2 | 100 | 100 | 3.5a | 60/150 | [24] |
| TiO2-CeO2 mixed oxides | 400 | 200 | 10.7a | 32/45 | [25] |
| SnO2/MoO2 | 100 | RT | 9.2%b | 20/16 | [1] |
| SnO2-CeO2 mixed oxides | 500 | 430 | 190%b | 26/30 | [11] |
| Pd/SnO2 nanowires | 200 | 400 | 6.8a | 5/40 | [26] |
| ZnO nanorods | 30 | 400 | 1.1a | 46/27 | [27] |
| Porous CeO2 nanosheets | 500 | 450 | 12.0%b | 2/2 | This work |
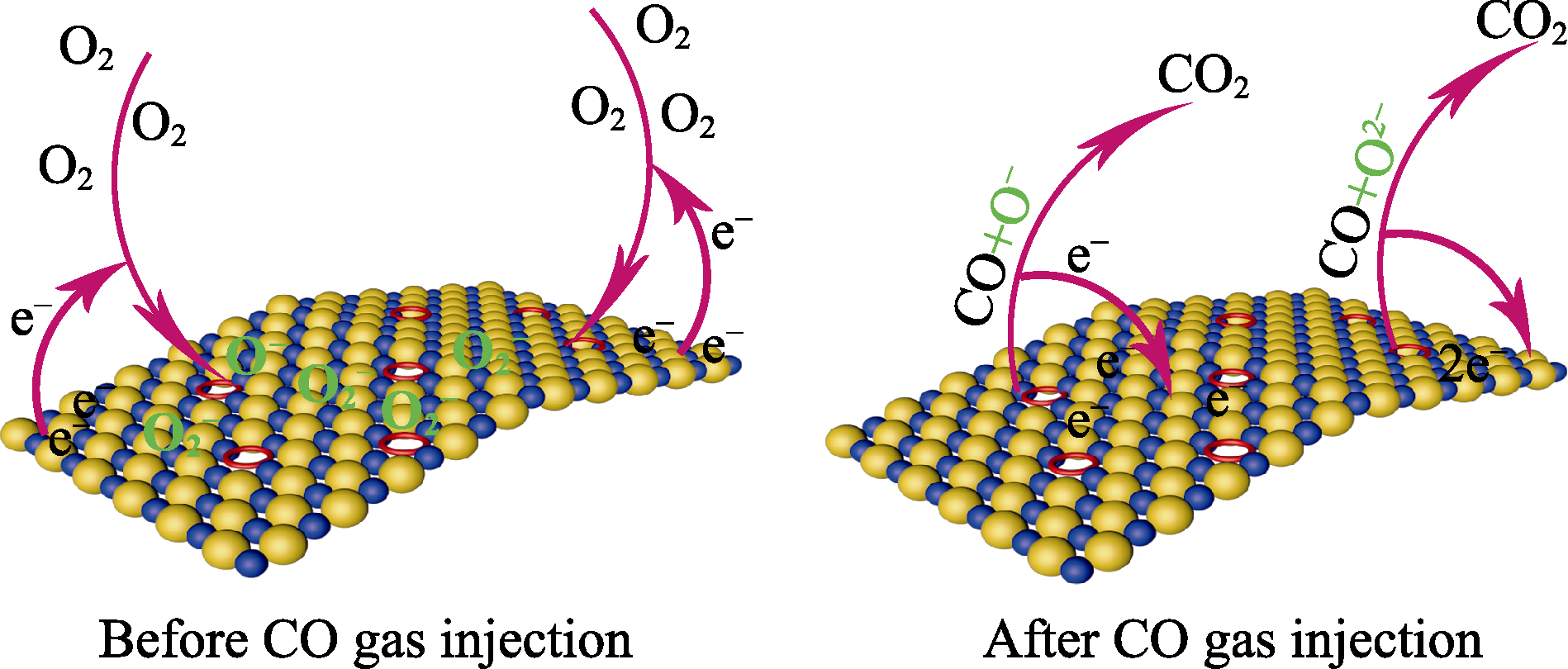
Fig. 8 Schematic illustration of CO gas sensing mechanism Yellow spheres represent O atoms; Orange spheres represent Ce atoms; Red rings represent the position of oxygen vacancies
| OS/% | OV/% | Ce3+/% | Ce3+/Ce4+ | |
|---|---|---|---|---|
| CeO2-0.5min NSs | 19.0 | 40.0 | 26.3 | 0.357 |
| CeO2-2min NSs | 14.8 | 43.2 | 28.6 | 0.401 |
| CeO2-5min NSs | 15.1 | 42.7 | 23.8 | 0.312 |
Table S1 Percentage of each element species to the whole element content
| OS/% | OV/% | Ce3+/% | Ce3+/Ce4+ | |
|---|---|---|---|---|
| CeO2-0.5min NSs | 19.0 | 40.0 | 26.3 | 0.357 |
| CeO2-2min NSs | 14.8 | 43.2 | 28.6 | 0.401 |
| CeO2-5min NSs | 15.1 | 42.7 | 23.8 | 0.312 |
| Surface area/(m2∙g-1) | Pore volume/ (cm3∙g-1) | Average pore diameter/nm | |
|---|---|---|---|
| CeO2-0.5min NSs | 73.344 | 0.172 | 10.1 |
| CeO2-2min NSs | 46.804 | 0.181 | 15.5 |
| CeO2-5min NSs | 40.836 | 0.163 | 15.8 |
Table S2 Summary of surface area and pore volume of CeO2 NSs
| Surface area/(m2∙g-1) | Pore volume/ (cm3∙g-1) | Average pore diameter/nm | |
|---|---|---|---|
| CeO2-0.5min NSs | 73.344 | 0.172 | 10.1 |
| CeO2-2min NSs | 46.804 | 0.181 | 15.5 |
| CeO2-5min NSs | 40.836 | 0.163 | 15.8 |
| [1] |
YANG Z, ZHANG D, WANG D. Carbon monoxide gas sensing properties of metal-organic frameworks-derived tin dioxide nanoparticles/molybdenum diselenide nanoflowers. Sensors and Actuators B: Chemical, 2019, 304:127369.
DOI URL |
| [2] |
BASU A K, CHAUHAN P S, AWASTHI M, et al. α-Fe2O3 loaded rGO nanosheets based fast response/recovery CO gas sensor at room temperature. Applied Surface Science, 2019, 465:56-66.
DOI URL |
| [3] | WHO. WHO Air Quality Guidelines-Global Update 2005. World Health Organization, Copenhagen, 2006. |
| [4] |
YANG S, JIANG C, WEI S H. Gas sensing in 2D materials. Applied Physics Reviews, 2017, 4(2):021304.
DOI URL |
| [5] |
DEY A. Semiconductor metal oxide gas sensors: a review. Materials Science and Engineering: B, 2018, 229:206-217.
DOI URL |
| [6] |
MAHAJAN S, JAGTAP S. Metal-oxide semiconductors for carbon monoxide (CO) gas sensing: a review. Applied Materials Today, 2020, 18:100483.
DOI URL |
| [7] |
GOVARDHAN K, GRACE A N. Metal/Metal oxide doped semiconductor based metal oxide gas sensors-a review. Sensor Letters, 2016, 14:741-750.
DOI URL |
| [8] | SUN C, LI H, CHEN L. Nanostructured ceria-based materials: synthesis, properties, and applications. Energy & Environmental Science, 2012, 5(9):8475-8505. |
| [9] |
LIU Y, LEI Y. Pt-CeO2 nanofibers based high-frequency impedancemetric gas sensor for selective CO and C3H8 detection in high- temperature harsh environment. Sensors and Actuators B: Chemical, 2013, 188:1141-1147.
DOI URL |
| [10] |
MAJUMDER D, ROY S. Development of low-ppm CO sensors using pristine CeO2 nanospheres with high surface area. ACS Omega, 2018, 3(4):4433-4440.
DOI URL |
| [11] |
DURRANI S M, AL-KUHAILI M F, BAKHTIARI I A, et al. Investigation of the carbon monoxide gas sensing characteristics of tin oxide mixed cerium oxide thin films. Sensors (Basel), 2012, 12(3):2598-2609.
DOI URL |
| [12] |
LIU X, MA T, PINNA N, et al. Two-dimensional nanostructured materials for gas sensing. Advanced Functional Materials, 2017, 27(37):1702168.
DOI URL |
| [13] |
LIU F, WANG X, CHEN X, et al. Porous ZnO ultrathin nanosheets with high specific surface areas and abundant oxygen vacancies for acetylacetone gas sensing. ACS Applied Materials Interfaces, 2019, 11(27):24757-24763.
DOI URL |
| [14] |
MIAO J, CHEN C, MENG L, et al. Self-assembled monolayer of metal oxide nanosheet and structure and gas-sensing property relationship. ACS Sensors, 2019, 4(5):1279-1290.
DOI URL |
| [15] |
CHOI P G, IZU N, SHIRAHATA N, et al. SnO2 nanosheets for selective alkene gas sensing. ACS Applied Nano Materials, 2019, 2(4):1820-1827.
DOI URL |
| [16] |
LI P, WANG B, QIN C, et al. Band-gap-tunable CeO2 nanoparticles for room-temperature NH3 gas sensors. Ceramics International, 2020, 46(11):19232-19240.
DOI URL |
| [17] |
LI P, WANG B, LI W, et al. Effect of annealing atmosphere with different oxygen concentration on CO gas sensing performances for CeO2 nanoparticles. Materials Letters, 2021, 284:129000.
DOI URL |
| [18] |
SAMERJAI T, CHANNEI D, KHANTA C, et al. Flame-spray- made ZnInO alloyed nanoparticles for NO2 gas sensing. Journal of Alloys and Compounds, 2016, 680:711-721.
DOI URL |
| [19] | YUAN H, ALJNEIBI S, YUAN J, et al. ZnO nanosheets abundant in oxygen vacancies derived from metal-organic frameworks for ppb-level gas sensing. Advanced Materials, 2019, 31(11):e1807161. |
| [20] |
ZHAO H, DONG Y, JIANG P, et al. Highly dispersed CeO2 on TiO2 nanotube: a synergistic nanocomposite with superior peroxidase-like activity. ACS Applied Materials Interfaces, 2015, 7(12):6451-6461.
DOI URL |
| [21] |
DENG C, HUANG Q, ZHU X, et al. The influence of Mn-doped CeO2 on the activity of CuO/CeO2 in CO oxidation and NO+CO model reaction. Applied Surface Science, 2016, 389:1033-1049.
DOI URL |
| [22] |
YAO L, LI Y, RAN Y, et al. Construction of novel Pd-SnO2 composite nanoporous structure as a high-response sensor for methane gas. Journal of Alloys and Compounds, 2020, 826:154063.
DOI URL |
| [23] |
BUSACCA C, DONATO A, LO FARO M, et al. CO gas sensing performance of electrospun Co3O4 nanostructures at low operating temperature. Sensors and Actuators B: Chemical, 2020, 303:127193.
DOI URL |
| [24] |
WANG Q, WANG C, SUN H, et al. Microwave assisted synthesis of hierarchical Pd/SnO2 nanostructures for CO gas sensor. Sensors and Actuators B: Chemical, 2016, 222:257-263.
DOI URL |
| [25] |
MOHAMMADI M R, FRAY D J. Nanostructured TiO2-CeO2 mixed oxides by an aqueous Sol-Gel process: effect of Ce:Ti molar ratio on physical and sensing properties. Sensors and Actuators B: Chemical, 2010, 150(2):631-640.
DOI URL |
| [26] |
TRUNG DO D, HOA N D, TONG P V, et al. Effective decoration of Pd nanoparticles on the surface of SnO2 nanowires for enhancement of CO gas-sensing performance. Journal of Hazard Materials, 2014, 265:124-132.
DOI URL |
| [27] |
KHOANG N D, HONG H S, TRUNG D D, et al. On-chip growth of wafer-scale planar-type ZnO nanorod sensors for effective detection of CO gas. Sensors and Actuators B: Chemical, 2013, 181:529-536.
DOI URL |
| [28] |
QIN C, WANG B, WU N,et al. Metal-organic frameworks derived porous Co3O4 dodecahedeons with abundant active Co3+ for ppb- level CO gas sensing. Applied Surface Science, 2020, 506:144900.
DOI URL |
| [29] |
XU J M, CHENG J P. The advances of Co3O4 as gas sensing materials: a review. Journal of Alloys and Compounds, 2016, 686:753-768.
DOI URL |
| [30] |
YAN F, SHEN G, YANG X, et al. Low operating temperature and highly selective NH3 chemiresistive gas sensors based on Ag3PO4 semiconductor. Applied Surface Science, 2019,479:1141-1147.
DOI URL |
| [31] | WANG Z, YU R. Hollow micro/nanostructured ceria-based materials: synthetic strategies and versatile applications. Advanced Materials, 2019,31(38):e1800592. |
| [1] | 周阳阳, 张艳艳, 于子怡, 傅正钱, 许钫钫, 梁瑞虹, 周志勇. 通过Bi3+自掺杂增强CaBi4Ti4O15基陶瓷压电性能[J]. 无机材料学报, 2025, 40(6): 719-728. |
| [2] | 孙雨萱, 王政, 时雪, 史颖, 杜文通, 满振勇, 郑嘹赢, 李国荣. 缺陷偶极子热稳定性对Fe掺杂PZT陶瓷机电性能影响研究[J]. 无机材料学报, 2025, 40(5): 545-551. |
| [3] | 范小暄, 郑永炅, 徐丽荣, 姚子敏, 曹硕, 王可心, 王绩伟. 基于富氧空位LiYScGeO4: Bi3+长余辉光催化剂的自激活余辉驱动有机污染物芬顿降解[J]. 无机材料学报, 2025, 40(5): 481-488. |
| [4] | 杜剑宇, 葛琛. 光电人工突触研究进展[J]. 无机材料学报, 2023, 38(4): 378-386. |
| [5] | 贾鑫, 李晋宇, 丁世豪, 申倩倩, 贾虎生, 薛晋波. Pd纳米颗粒协同氧空位增强TiO2光催化CO2还原性能[J]. 无机材料学报, 2023, 38(11): 1301-1308. |
| [6] | 李豪, 唐志红, 卓尚军, 钱荣. 基于ZIF8/rGO的高性能NO2室温传感器[J]. 无机材料学报, 2021, 36(12): 1277-1282. |
| [7] | 张文进, 申倩倩, 薛晋波, 李琦, 刘旭光, 贾虎生. 具有高度有序氧空位的α-Fe2O3纳米带的制备及光电催化水氧化性能研究[J]. 无机材料学报, 2021, 36(12): 1290-1296. |
| [8] | 刘亚鑫, 王敏, 沈梦, 王强, 张玲霞. 铋掺杂提高氧化铈中氧空位浓度增强CO2光催化还原性能[J]. 无机材料学报, 2021, 36(1): 88-94. |
| [9] | 伍凡, 赵梓俨, 黎邦鑫, 董帆, 周莹. Bi2O2CO3/PPy界面氧空位构建及其可见光下NO氧化机理研究[J]. 无机材料学报, 2020, 35(5): 541-548. |
| [10] | 胡浩, 江向平, 陈超, 聂鑫, 黄枭坤, 苏春阳. Ce 3+掺杂Na0.5Bi8.5Ti7O27铋层状陶瓷的结构与电性能研究[J]. 无机材料学报, 2019, 34(9): 997-1003. |
| [11] | 李金, 刘廷禹, 姚舒安, 付明雪, 鲁晓晓. 第一原理研究LuPO4中氧空位的性质[J]. 无机材料学报, 2019, 34(8): 879-884. |
| [12] | 崔 磊, 杨丽娟, 王 帆, 夏炜炜. 花状空心Sn3O4微球的制备及其光催化性能的研究[J]. 无机材料学报, 2016, 31(5): 461-465. |
| [13] | 赵 倩, 吴 萍. 缺陷诱导的Cr掺杂TiO2纳米粉末的铁磁性[J]. 无机材料学报, 2013, 28(10): 1098-1102. |
| [14] | 杨天天, 许鹏程, 左国民, 李昕欣. 自组装单层膜模板控制生长氧化亚铜晶体及其对DMMP气体的检测[J]. 无机材料学报, 2011, 26(10): 1111-1115. |
| [15] | 周志刚,唐子龙. 化学传感器陶瓷点缺陷及其应用[J]. 无机材料学报, 2009, 24(4): 650-660. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||